Abstract
Testicular torsion results with the damage of the testis and it is a surgical emergency. Pyrrolidine dithiocarbamate (PDTC) is a low-molecular-weight antioxidant and potent inhibitor of nuclear factor kappa B (NF-κB) activation. In this study, we aimed to investigate the effects of PDTC to testicular torsion-detorsion (T/D) injury. Forty adult male Sprague-Dawley rats were separated into four groups. A sham operation was performed in group I. In group II, torsion is performed 2 hours by 720 degree extravaginally testis. In group III, 4 h reperfusion of the testis was performed after 2 h of testicular torsion. In group IV, after performing the same surgical procedures as in group III, PDTC (100 mg/kg, intravenous's) was administered before 30 min of detorsion. The testes tissue malondialdehyde (MDA), superoxide dismutase (SOD) catalase (CAT) level was evaluated. Histological evaluations were performed after hematoxylin and eosin staining. Testicular tissue MDA levels were the highest in the T/D groups compared with treatment group. Administration of PDTC prevented a further increase in MDA levels. Significant decrease occurred in CAT and SOD levels in treatment group compared with the control group. The rats in the treatment group had normal testicular architecture. The results suggest that PDTC can be a potential protective agent for preventing the biochemical and histological changes related to oxidative stress in testicular injury caused by testis torsion.
Testicular torsion is an urologic emergency that affects newborns, children and adolescents. This condition also requires immediate surgical intervention to prevent testicular damage [1]. Testicular torsion leads to altered hormone production, and subfertility and infertility [2]. Ischemia and reperfusion (I/R) injury leads to the production of excessive reactive oxygen species (ROS), which can initiate lipid peroxidation, and oxidise proteins to inactive states and cause DNA strand breaks [3,4]. It has been known that during ischemia, cells and tissues undergo rapid changes which lead to perturbations in signaling pathways and surface molecule expression. These events are thought to contribute to the tissue damage during I/R in various organs. The main cause of testicular damage after torsion is ischemia followed by reperfusion, which are known to have deleterious consequences of I/R [5]. The ischemic tissue leads to a complex cascade of events that includes the activation of nuclear factor kappa β (NF-κB), which controls cytokine, chemokines and adhesion molecules [6]. Previously, many preventative pretreatment agents like antioxidants and ROS scavengers were shown in testicular torsion/detorsion (T/D) experimental models. However, the molecular mechanism of antioxidants which control testicular torsion-induced male fertility has not yet been clearly identified [7]. Pyrrolidine dithiocarbamate (PDTC) is a low-molecular weight thiol compound. PDTC has different properties such as, redox state alternation, heavy metal chelation, and enzyme inhibition [8,9]. Studies have suggested that potent inhibitor of NF-κB which used as an antioxidant compound to counteract the toxic effects of free radicals and to interfere with the generation of proinflammatory cytokines [10,11]. Although studies showed that the protective effects of PDTC might inhibit NF-κB activation, PDTC has a potential to activate gene expression of endogenous antioxidants and independent of any effects on NF-κB [12,13]. In addition, some studies suggested that the protective effects of PDTC might inhibit NF-κB activation via stabilization of IκB-α and inhibit of the ubiquitin-proteasome pathway [14,15]. Also, it has been demonstrated that PDTC is one of the most effective inducers of heme oxygenase-1 (HO-1), which also provides cytoprotection against oxidative stress [13,16].
The aim of the study is to evaluate the effects of PDTC on testicular ischemia reperfusion injury, by determining biochemical parameters and evaluating histological examinations.
The experimental protocol was approved by the institutional animal ethics committee. Forty adult male Sprague-Dawley rats weighting 220 to 250 g were obtained from Medical and Surgical Experimental Research Center (Eskisehir-Turkey). Rats were housed in polycarbonate cages in a room with controlled temperature (22±2℃), humidity (50±5%), and a 12 h. cycle of light and dark and were fed laboratory pellet chows and water was given water ad libitum. The experiment was performed after a stabilization period in the laboratory for five days.
The surgical procedures were done under general anesthesia induced by intraperitoneal injection of ketamine HCl (50 mg/kg) and chlorpromazine (25 mg/kg). The skin of the scrotal area was shaved and prepared with 10% povidone iodine solution. A mid-scrotal vertical incision was performed for access to both testes. Torsion was created by twisting the right testis 720° in a counterclockwise direction and maintained by fixing the testis to the scrotum with a 5-0 nylon suture passing through the tunica albuginea and dartos. After 2 hour of ischemia, the suture was removed, and the right testis was untwisted and replaced in the scrotum for 4 hours of reperfusion. During the sham operation, the right testis was brought through the incision and then replaced without twisting, and a nylon suture was placed through the tunica albuginea. After each surgical intervention, the incision was closed. The animals were decapitated at the end of the experiment. Bilateral orchiectomies were performed for histologic examination and measurement of tissue malondialdehyde (MDA), superoxide dismutase (SOD), and catalase (CAT) levels.
The rats were randomly separated into four equal groups (10 animals each). Group I (sham-operated control group) underwent a sham operation to determine basal values for biochemical and histopathological evaluation. The testes were brought through the incision and then replaced with a fixation to the scrotum with no additional intervention. Group II (torsion) was designed to study the effects of testicular torsion. In this group, torsion is performed for 2 hours by 720 degree extravaginally testis. Group III (torsion/detorsion) was designed to study the effect of detorsion after testicular torsion. In this group, 4 h reperfusion of the testis was performed after 2 h of testicular torsion. Group IV (torsion-PDTC treatment-detorsion) was designed to determine the effect of PDTC after torsion. After performing the same surgical procedures as in group III, PDTC (100 mg/kg, intravenous's) (Dithiocarbamate; Sigma-Aldrich, Steinheim, Germany) was administered before 30 min of detorsion.
At the end of the surgical procedure, testicular specimens were individually immersed in Bouin's fixative (7.5 ml of saturated picric acid, 2.65 ml of glacial acetic acid and 2.5 ml of 7% formaldehyde) and they were dehydrated in alcohol and embedded in paraffin. Five micrometer sections were obtained, deparaffinized and stained with haematoxylin and eosin (H&E). The testicular tissue was evaluated in random order with standard light microscopy (NIKON, Japan) by an observer unaware of which group the rat had belonged. Three slides prepared from the upper, lower and midportions of the testes were evaluated completely for each testis.
After sacrificing the animals, testes were quickly removed and perfused immediately with ice-cold normal saline, and homogenized in chilled potassium chloride (1.17%). The homogenate was centrifuged at 800×g for 5 min at 4℃ to separate the nuclear debris. The supernatant so obtained was centrifuged at 10,500×g for 20 min at 4℃ to get the homogenate which was used to assay MDA, CAT, and SOD activity.
MDA production was an end product of lipid peroxidation reacts with thiobarbituric acid to form a red colored complex. 0.1 ml of homogenate, 3 ml of 1% phosphoric acid, 0.5 ml of distilled water and 1.0 ml of 0.6% 2-thiobarbituric acid were added. The mixture was boiled in water bath for 45 min, followed by cooling in an ice, and addition of 4.0 ml of n-butanol to extract the cold thiobarbituric acid reactants. The optical density of the n-butanol layer was determined at 532 nm after centrifugation at 1,000 g for five minutes and expressed as nmol MDA/g of wet tissue [17].
SOD activity was spectrophotometrically assayed with commercial kits (Fluka SOD kit USA). It is an indirect assay method based on xanthine oxidase and a novel color reagent. SOD activity in the homogenate was determined by inhibition of Formosan dye (450 nm) employing the xanthin-xanthin oxidase enzymatic method to generate superoxide radicals and calculated the active SOD concentration according to inhibition curve graphic expressed as U/g of wet tissue.
One unit (1U) of CAT equals the enzyme activity that recognized 1 µmol of hydrogen peroxide in 60 s at 37℃. CAT activity was measured with determination of absorbance of three blank samples at 405 nm. CAT activity (kU/L) was calculated as=[(Absblank1-Absblanksample)/Absblank2-Absblank3)]×271 [18].
All statistical analysis was performed with the computer program "SPSS for Windows" (SPSS Inc; Release 11.5; Sep 6, 2002). All of the data were expressed as means±SD. Differences between groups were evaluated by one-way analysis of variance (ANOVA) followed by Tukey's multiple comparison tests. The significance was tested at p>0.05, p<0.05, p<0.01 and p<0.001.
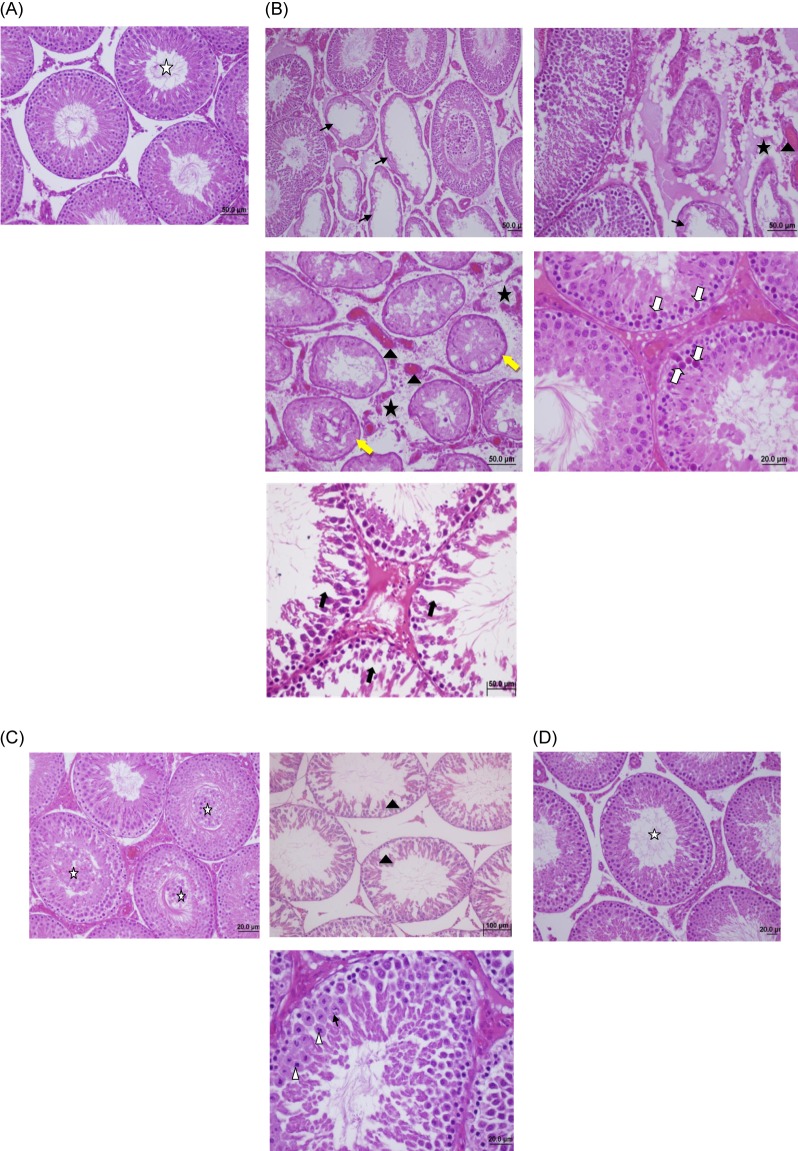
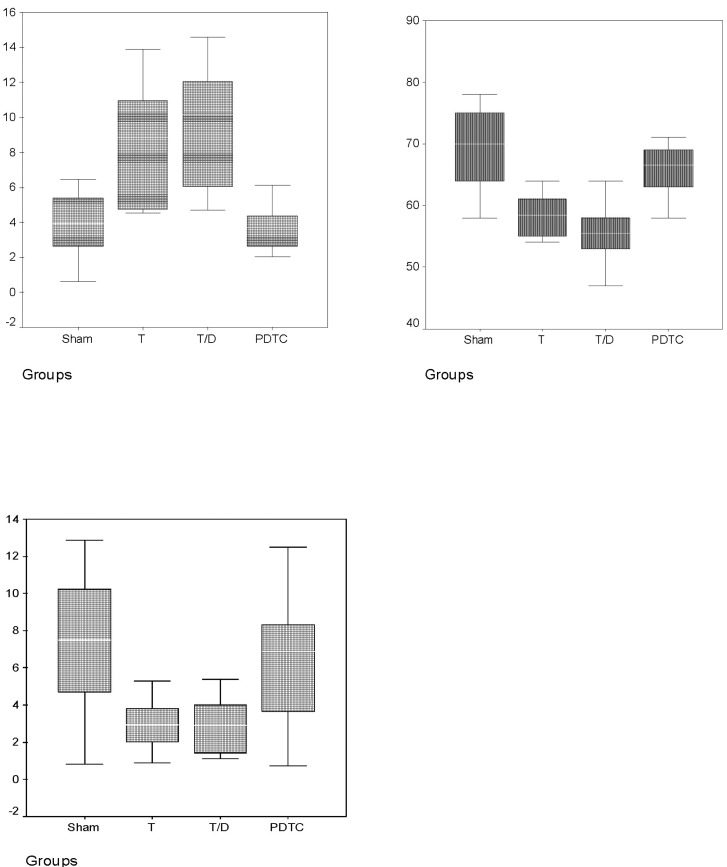
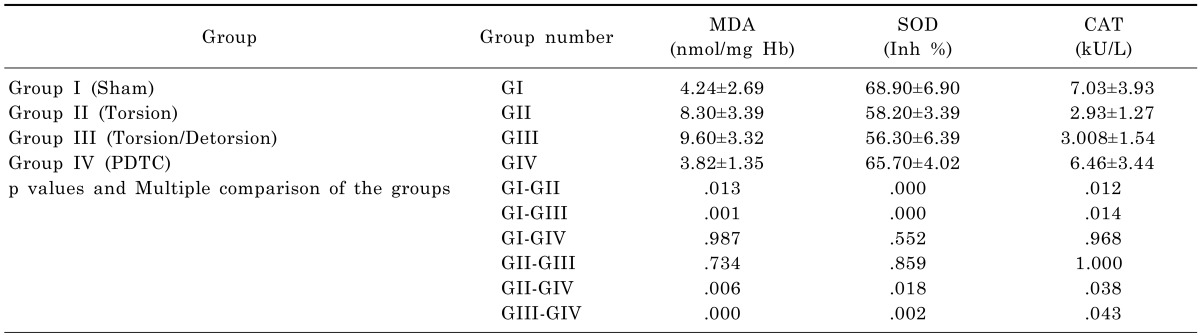
The values of MDA, SOD and CAT measurements for the different groups are shown in Table 1 and Fig. 1. The MDA levels of group II and III in testes tissue were significantly higher than group I and group IV. PDTC administration had significantly decreased MDA levels in group IV when compared with group II and III (p<0.05). But, there was no significant difference between the group II and group III (p>0.05). The SOD and CAT levels were significantly decreased in group II and III when compared with group I and IV, but after PDTC administration SOD and CAT levels increased and there weren't any difference between group I and IV (p>0.05). Also these results suggested that PDTC may be effective in preventing oxidative injury. The findings of the histopathological evaluation for each group were shown in Fig. 2. The testes of rats in sham-operated control group showed the presence of normal testicular architecture and regular seminiferous tubular morphology with normal spermatogenesis (Fig. 2A). In Group II (ischemia) severe tubular degeneration was observed, many tubule of spermatogenic cell lines completely disappeared, also thickening of the basal laminae of tubules, interstitial edema and vascular congestions in the area were seen, and some of the tubules necrotic (pyknotic nuclei and eosinophilic cytoplasm) cells spermatocytes, which was more pronounced with detorsion (Fig. 2B). In addition, in the T/D group we observed closed tubule lumen with many cellular desquamation, degeneration of germ cells, severe tubular degeneration necrotic cells and eosinophilic accumulation of fluid in the interstitial space and vascular congestions (Fig. 2C). The PDTC-treated group showed maturation up to the level of spermatozoa, with preservation of tubular morphology (Fig. 2D). Administration of PDTC caused significant rescue of testicular function by preserving the intact seminiferous tubular morphology.
Testicular torsion and detorsion leads to biochemical and morphologic changes caused by ischemia-reperfusion (IR) injury in the testicular tissue [19]. Various pathogenic mechanisms have been reported to explain the tissue damage that occurs during testicular torsion and detorsion such as testicular lipid peroxidation, vascular leukocyte margination and apoptosis, which in turn leads to membrane damage [4]. The detorsion involves the production of toxic reactive oxygen species (ROS) with the return of blood flow following ischemia [5]. Testicular torsion and detorsion injury decrease also frees radical scavengers (SOD and CAT), as shown in our study. Thus, defining the activities of free radical scavenger enzymes in testicular tissue provides some important clues about free radical formation. Excessive amounts of oxygen free radicals cause membrane lipid peroxidation which can be measured by tissue MDA levels and it is a well-known parameter for determining the raised free radical formation in reperfused tissue [17]. In our study, the level of MDA significantly increased in the T/D group when compared to the sham group. Treatment with PDTC significantly decreased MDA levels. Histologically, I/R induced tubular degeneration, congestion, interstitial oedema and hemorrhage in the rat testis. These findings show that PDTC-treated group had preserved normal testicular architecture and regular seminiferous tubular morphology with normal spermatogenesis.
Recent studies have shown that ROS activate the NF-kB family of transcription factors, which controls the formation of proinflammatory cytokines (e.g., tumor necrosis factor-α, interleukin-1β, interleukin-6, or interleukin-8), the expression on endothelium and neutrophils of adhesion molecules (e.g. vascular cell adhesion molecule-1, intercellular adhesion molecule-1), and the overproduction of vasoactive mediators (e.g., NO by inducible nitric oxide synthase (iNOS) or eicosanoids via cyclo-oxygenase-2). This complex cascade plays important role for the pathophysiology of reperfusion injury [6,20,21]. Previous studies have demonstrated the role of NF-kB in injuries of organs such as the kidney, liver pancreas and nerve injury [21,22,23,24,25]. PDTC is also a chelator of heavy metals and this capacity probably prevents formation of OH- radicals produced through the Haber-Weiss reaction. Several investigators reported the reduction in both renal and testicular lipid peroxidation in animals treated with dithiocarbamates following challenge with a variety of heavy metals [26,27]. Generally, antioxidants have been shown their effects via a direct toxic action on target cells. In gene induction, it is thought that oxidants may play a contributory role. Low levels of ROS activate NF-kB, while antioxidants inhibit NF-kB which is a pleiotropic transcription factor [28]. PDTC which is thought as a potent inhibitor of NF-kB can be used as an antioxidant against the toxic effects of free radicals and generation of proinflammatory cytokines [10,29,30]. It is suggested that PDTC has a potential to activate gene expression of endogenous antioxidants, such as superoxide dismutase [12,13]. PDTC has been shown to increase the cytotoxicity of the chemotherapeutic agent 5-fluorouracil in animal models of colorectal cancer [31]. PDTC has also been representing to induce proapoptotic and antiproliferative effects in prostate cancer, renal cancer and leukemia [32,33,34]. Moreover, PDTC has been demonstrated to induce apoptosis in tumor cells by inhibiting the proteasomal activity [35]. Chae et al demonstrated that PDTC has an inhibitory effect on TNF-α-mediated activation of JNK/SAPK, AP-1, cytochrome c release and subsequent caspase-3, because of its effect of the inhibition of apoptosis. They suggested that it may be a theuropatic option for the TNF-α-associated immune and inflammatory diseases such as rheumatoid arthritis and periodontal diseases [36]. Zhang et al showed that PDTC potent anticancer activity against cisplatin-resistant neuroblastoma cells [37]. Recently, it has been demonstrated in an animal model that PDTC markedly ameliorated renal, brain, intestinal, mesenteric, colonic, liver, lung and gastric I-R injury [38,39,40,41,42,43,44,45]. PDTC has been shown to potent protective effect against the testicular damage of cisplatin-induced testicular toxicity and demonstrate that blockade of NF-kB activation by an antioxidant could be an effective strategy for prophylaxis of cisplatin-induced testicular damage [46].
In conclusion, the present study demonstrates that PDTC prevents testicular T/D injury induced biochemical and histologic changes testicular tissues in the rat. The clinical implications of these results merits further experimental and clinical studies to be performed.
References
2. Anderson JB, Williamson RC. Testicular torsion in Bristol: a 25-year review. Br J Surg. 1988; 75:988–992. PMID: 3219547.

3. Ames BN. Endogenous oxidative DNA damage, aging, and cancer. Free Radic Res Commun. 1989; 7:121–128. PMID: 2684796.

4. de Groot H, Rauen U. Ischemia-reperfusion injury: processes in pathogenetic networks: a review. Transplant Proc. 2007; 39:481–484. PMID: 17362763.

5. Granger DN, Korthuis RJ. Physiologic mechanisms of postischemic tissue injury. Annu Rev Physiol. 1995; 57:311–332. PMID: 7778871.

6. Yang S, Shih HJ, Chow YC, Tsai PS, Huang CJ. Hemin induced heme oxygenase-1 over expression involves nuclear factor-E2 related factor-2, nuclear factor-kappaB and extracellular regulated kinase: an experimental study in a testicular torsion-detorsion rodent model. J Urol. 2008; 179:2456–2463. PMID: 18433787.
7. Altavilla D, Romeo C, Squadrito F, Marini H, Morgia G, Antonuccio P, Minutoli L. Molecular pathways involved in the early and late damage induced by testis ischemia: evidence for a rational pharmacological modulation. Curr Med Chem. 2012; 19:1219–1224. PMID: 22300051.
8. Orrenius S, Nobel CS, van den Dobbelsteen DJ, Burkitt MJ, Slater AF. Dithiocarbamates and the redox regulation of cell death. Biochem Soc Trans. 1996; 24:1032–1038. PMID: 8968507.

9. Iseki A, Kambe F, Okumura K, Niwata S, Yamamoto R, Hayakawa T, Seo H. Pyrrolidine dithiocarbamate inhibits TNF-alpha-dependent activation of NF-kappaB by increasing intracellular copper level in human aortic smooth muscle cells. Biochem Biophys Res Commun. 2000; 276:88–92. PMID: 11006087.
10. Liu SF, Ye X, Malik AB. Inhibition of NF-kappaB activation by pyrrolidine dithiocarbamate prevents In vivo expression of proinflammatory genes. Circulation. 1999; 100:1330–1337. PMID: 10491379.
11. Bowie A, O'Neill LA. Oxidative stress and nuclear factor-kappaB activation: a reassessment of the evidence in the light of recent discoveries. Biochem Pharmacol. 2000; 59:13–23. PMID: 10605930.
12. Schreck R, Meier B, Männel DN, Dröge W, Baeuerle PA. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J Exp Med. 1992; 175:1181–1194. PMID: 1314883.

13. Borrello S, Demple B. NF kappa B-independent transcriptional induction of the human manganous superoxide dismutase gene. Arch Biochem Biophys. 1997; 348:289–294. PMID: 9434740.
14. Liu SF, Ye X, Malik AB. In vivo inhibition of nuclear factor-kappa B activation prevents inducible nitric oxide synthase expression and systemic hypotension in a rat model of septic shock. J Immunol. 1997; 159:3976–3983. PMID: 9378986.
15. Si X, McManus BM, Zhang J, Yuan J, Cheung C, Esfandiarei M, Suarez A, Morgan A, Luo H. Pyrrolidine dithiocarbamate reduces coxsackievirus B3 replication through inhibition of the ubiquitin-proteasome pathway. J Virol. 2005; 79:8014–8023. PMID: 15956547.

16. Hartsfield CL, Alam J, Choi AM. Transcriptional regulation of the heme oxygenase 1 gene by pyrrolidine dithiocarbamate. FASEB J. 1998; 12:1675–1682. PMID: 9837857.

17. Mihara M, Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978; 86:271–278. PMID: 655387.
18. Góth L. A simple method for determination of serum catalase activity and revision of reference range. Clin Chim Acta. 1991; 196:143–151. PMID: 2029780.

19. Saba M, Morales CR, De Lamirande E, Gagnon C. Morphological and biochemical changes following acute unilateral testicular torsion in prepubertal rats. J Urol. 1997; 157:1149–1154. PMID: 9072560.

20. Németh ZH, Haskó G, Vizi ES. Pyrrolidine dithiocarbamate augments IL-10, inhibits TNF-alpha, MIP-1alpha, IL-12, and nitric oxide production and protects from the lethal effect of endotoxin. Shock. 1998; 10:49–53. PMID: 9688091.
21. Sun Z, Andersson R. NF-kappaB activation and inhibition: a review. Shock. 2002; 18:99–106. PMID: 12166787.
22. Frossard JL, Pastor CM, Hadengue A. Effect of hyperthermia on NF-kappaB binding activity in cerulein-induced acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2001; 280:G1157–G1162. PMID: 11352808.
23. Orfila C, Lepert JC, Alric L, Carrera G, Béraud M, Pipy B. Immunohistochemical distribution of activated nuclear factor kappaB and peroxisome proliferator-activated receptors in carbon tetrachloride-induced chronic liver injury in rats. Histochem Cell Biol. 2005; 123:585–593. PMID: 15959796.
24. Tugcu V, Ozbek E, Tasci AI, Kemahli E, Somay A, Bas M, Karaca C, Altug T, Cekmen MB, Ozdogan HK. Selective nuclear factor kappa-B inhibitors, pyrolidium dithiocarbamate and sulfasalazine, prevent the nephrotoxicity induced by gentamicin. BJU Int. 2006; 98:680–686. PMID: 16925772.
25. Gu EY, Han HS, Park JS. Effect of minocycline on activation of glia and nuclear factor kappa B in an animal nerve injury model. Korean J Physiol Pharmacol. 2004; 8:237–243.
26. Hidaka S, Funakoshi T, Shimada H, Tsuruoka M, Kojima S. Protective effect of N-benzyl-D-glucamine dithiocarbamate against renal toxicity in rats during repeated cis-diamminedichloroplatinum administrations. Ren Fail. 1995; 17:539–550. PMID: 8570866.
27. Kojima S, Sugimura Y, Ono H, Shimada H, Funakoshi T. N-benzyl-D-glucamine dithiocarbamate and N-p-isopropylbenzyl-D-glucamine dithiocarbamate improve the protective effect of diethyldithiocarbamate against cadmium-induced testicular toxicity in rats. Biol Pharm Bull. 1993; 16:244–247. PMID: 8395932.

28. Schwartz MD, Moore EE, Moore FA, Shenkar R, Moine P, Haenel JB, Abraham E. Nuclear factor-kappa B is activated in alveolar macrophages from patients with acute respiratory distress syndrome. Crit Care Med. 1996; 24:1285–1292. PMID: 8706481.

29. Li C, Browder W, Kao RL. Early activation of transcription factor NF-kappaB during ischemia in perfused rat heart. Am J Physiol. 1999; 276:H543–H552. PMID: 9950856.
30. Muller DN, Dechend R, Mervaala EM, Park JK, Schmidt F, Fiebeler A, Theuer J, Breu V, Ganten D, Haller H, Luft FC. NF-kappaB inhibition ameliorates angiotensin II-induced inflammatory damage in rats. Hypertension. 2000; 35:193–201. PMID: 10642297.
31. Bach SP, Chinery R, O'Dwyer ST, Potten CS, Coffey RJ, Watson AJ. Pyrrolidinedithiocarbamate increases the therapeutic index of 5-fluorouracil in a mouse model. Gastroenterology. 2000; 118:81–89. PMID: 10611156.

32. Chen D, Peng F, Cui QC, Daniel KG, Orlu S, Liu J, Dou QP. Inhibition of prostate cancer cellular proteasome activity by a pyrrolidine dithiocarbamate-copper complex is associated with suppression of proliferation and induction of apoptosis. Front Biosci. 2005; 10:2932–2939. PMID: 15970547.

33. Morais C, Pat B, Gobe G, Johnson DW, Healy H. Pyrrolidine dithiocarbamate exerts anti-proliferative and pro-apoptotic effects in renal cell carcinoma cell lines. Nephrol Dial Transplant. 2006; 21:3377–3388. PMID: 16998220.

34. Arima N, Arimura K, Tokito Y, Sakaki Y, Matsushita K, Orihara K, Akimoto M, Ozaki A, Kukita T, Hagiwara T, Hamada H, Tei C. HTLV-I Tax protein inhibits apoptosis induction but not G1 arrest by pyrrolidinedithiocarbamate, an anti-oxidant, in adult T cell leukemia cells. Exp Hematol. 2004; 32:195–201. PMID: 15102481.

35. Milacic V, Chen D, Giovagnini L, Diez A, Fregona D, Dou QP. Pyrrolidine dithiocarbamate-zinc(II) and -copper(II) complexes induce apoptosis in tumor cells by inhibiting the proteasomal activity. Toxicol Appl Pharmacol. 2008; 231:24–33. PMID: 18501397.

36. Chae HJ, Bae J, Chae SW. PDTC Inhibits TNF-α-Induced Apoptosis in MC3T3E1 Cells. Korean J Physiol Pharmacol. 2003; 7:199–206.
37. Zhang H, Wu JS, Peng F. Potent anticancer activity of pyrrolidine dithiocarbamate-copper complex against cisplatin-resistant neuroblastoma cells. Anticancer Drugs. 2008; 19:125–132. PMID: 18176108.

38. Chatterjee PK, di Villa Bianca RD, Sivarajah A, McDonald MC, Cuzzocrea S, Thiemermann C. Pyrrolidine dithiocarbamate reduces renal dysfunction and injury caused by ischemia/reperfusion of the rat kidney. Eur J Pharmacol. 2003; 482:271–280. PMID: 14660032.

39. Li J, Sheng W, Feng C, Zuo Z. Pyrrolidine dithiocarbamate attenuates brain Aβ increase and improves long-term neurological outcome in rats after transient focal brain ischemia. Neurobiol Dis. 2012; 45:564–572. PMID: 21983158.

40. Teke Z, Kabay B, Aytekin FO, Yenisey C, Demirkan NC, Sacar M, Erdem E, Ozden A. Pyrrolidine dithiocarbamate prevents 60 minutes of warm mesenteric ischemia/reperfusion injury in rats. Am J Surg. 2007; 194:255–262. PMID: 17618816.

41. Teke Z, Aytekin FO, Kabay B, Yenisey C, Aydin C, Tekin K, Sacar M, Ozden A. Pyrrolidine dithiocarbamate prevents deleterious effects of remote ischemia/reperfusion injury on healing of colonic anastomoses in rats. World J Surg. 2007; 31:1835–1842. PMID: 17566823.

42. Mallick IH, Yang WX, Winslet MC, Seifalian AM. Pyrrolidine dithiocarbamate reduces ischemia-reperfusion injury of the small intestine. World J Gastroenterol. 2005; 11:7308–7313. PMID: 16437633.

43. Tian XF, Yao JH, Li YH, Gao HF, Wang ZZ, Yang CM, Zheng SS. Protective effect of pyrrolidine dithiocarbamate on liver injury induced by intestinal ischemia-reperfusion in rats. Hepatobiliary Pancreat Dis Int. 2006; 5:90–95. PMID: 16481291.
44. Kabay B, Teke Z, Aytekin FO, Yenisey C, Bir F, Sacar M, Erdem E, Ozden A. Pyrrolidine dithiocarbamate reduces lung injury caused by mesenteric ischemia/reperfusion in a rat model. World J Surg. 2007; 31:1707–1715. PMID: 17551782.

45. El Eter E, Hagar HH, Al-Tuwaijiri A, Arafa M. Nuclear factor-kappaB inhibition by pyrrolidinedithiocarbamate attenuates gastric ischemia-reperfusion injury in rats. Can J Physiol Pharmacol. 2005; 83:483–492. PMID: 16049548.
46. Ilbey YO, Ozbek E, Simsek A, Cekmen M, Otunctemur A, Somay A. Chemoprotective effect of a nuclear factor-kappaB inhibitor, pyrrolidine dithiocarbamate, against cisplatin-induced testicular damage in rats. J Androl. 2009; 30:505–514. PMID: 19234314.
Fig. 2
(A) Sham group shows normal testicular architecture and regular seminiferous tubular morphology with normal spermatogenesis (star) (H&E, 50×). (B) Torsion group shows severe tubular degeneration, many of the tubules of spermatogenic cell lines completely disappeared (black arrows), thickening of the basal laminae of tubules (yellow arrows), Interstitial edema (stars) and vascular congestions, hemorrhage (black arrowhead) in the area, some of the tubules has necrotic (pyknotic nuclei and eosinophilic cytoplasm) cells spermatocytes (white arrows) (H&E, 20×, 50×). (C) Torsion/Detorsion group shows tubule lumen be filled with many cellular desquamation, degeneration of germ cells (stars), severe tubular degeneration (black arrowhead), necrotic cells (white arrowhead) and eosinophilic accumulation of fluid in the interstitial space and vascular congestions (black arrows) (H&E, 20×, 100×). (D) The PDTC-treated group shows maturation up to the level of spermatozoa, with preservation of tubular morphology (H&E, 20×).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download