1. Nestle FO, Di Meglio P, Qin JZ, Nickoloff BJ. Skin immune sentinels in health and disease. Nat Rev Immunol. 2009; 9:679–691. PMID:
19763149.

2. Cho YS, Lee KH, Park JW. Pyrithione-zinc Prevents UVB-induced Epidermal Hyperplasia by Inducing HIF-1alpha. Korean J Physiol Pharmacol. 2010; 14:91–97. PMID:
20473380.
3. Yamaguchi Y, Brenner M, Hearing VJ. The regulation of skin pigmentation. J Biol Chem. 2007; 282:27557–27561. PMID:
17635904.

4. Aoki Y, Tanigawa T, Abe H, Fujiwara Y. Melanogenesis inhibition by an oolong tea extract in b16 mouse melanoma cells and UV-induced skin pigmentation in brownish guinea pigs. Biosci Biotechnol Biochem. 2007; 71:1879–1885. PMID:
17690471.

5. Kim SY, Na JI, Park SJ, Choi HR, Kim DS, Park KC. Novel tri-peptides with hypopigmenting activity. J Dermatol Sci. 2012; 65:68–69. PMID:
22154815.

6. Costin GE, Hearing VJ. Human skin pigmentation: melanocytes modulate skin color in response to stress. FASEB J. 2007; 21:976–994. PMID:
17242160.

7. Hearing VJ, Tsukamoto K. Enzymatic control of pigmentation in mammals. FASEB J. 1991; 5:2902–2909. PMID:
1752358.

8. Buscà R, Ballotti R. Cyclic AMP a key messenger in the regulation of skin pigmentation. Pigment Cell Res. 2000; 13:60–69. PMID:
10841026.
9. Kim DS, Park SH, Kwon SB, Park ES, Huh CH, Youn SW, Park KC. Sphingosylphosphorylcholine-induced ERK activation inhibits melanin synthesis in human melanocytes. Pigment Cell Res. 2006; 19:146–153. PMID:
16524430.

10. Kim DS, Hwang ES, Lee JE, Kim SY, Kwon SB, Park KC. Sphingosine-1-phosphate decreases melanin synthesis via sustained ERK activation and subsequent MITF degradation. J Cell Sci. 2003; 116:1699–1706. PMID:
12665551.

11. Faller C, Bracher M, Dami N, Roguet R. Predictive ability of reconstructed human epidermis equivalents for the assessment of skin irritation of cosmetics. Toxicol In Vitro. 2002; 16:557–572. PMID:
12206823.

12. Kim J, Jeong HS, Li H, Baek KJ, Kwon NS, Yun HY, Choi HR, Park KC, Kim DS. Effects of Cervi cornus Colla (deer antler glue) in the reconstruction of a skin equivalent model. Arch Dermatol Res. 2013; 305:85–89. PMID:
23011660.

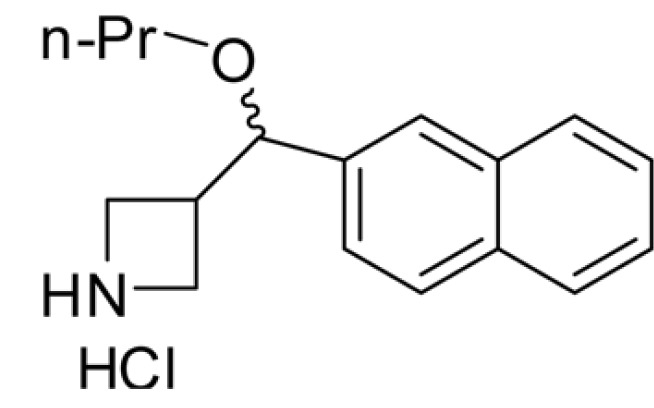
13. Han Y, Han M, Shin D, Song C, Hahn HG. Exploration of novel 3-substituted azetidine derivatives as triple reuptake inhibitors. J Med Chem. 2012; 55:8188–8192. PMID:
22938049.

14. Dooley TP, Gadwood RC, Kilgore K, Thomasco LM. Development of an in vitro primary screen for skin depigmentation and antimelanoma agents. Skin Pharmacol. 1994; 7:188–200. PMID:
8024800.

15. Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988; 106:761–771. PMID:
2450098.

16. Tsuboi T, Kondoh H, Hiratsuka J, Mishima Y. Enhanced melanogenesis induced by tyrosinase gene-transfer increases boron-uptake and killing effect of boron neutron capture therapy for amelanotic melanoma. Pigment Cell Res. 1998; 11:275–282. PMID:
9877098.

17. Auger FA, López Valle CA, Guignard R, Tremblay N, Noël B, Goulet F, Germain L. Skin equivalent produced with human collagen. In Vitro Cell Dev Biol Anim. 1995; 31:432–439. PMID:
8589886.

18. del Marmol V, Beermann F. Tyrosinase and related proteins in mammalian pigmentation. FEBS Lett. 1996; 381:165–168. PMID:
8601447.

19. Kim SY, Hahn HG, Nam KD, Park KC, Yun HY, Baek KJ, Kwon NS, Kim DS. A derivative of 2-aminothiazole inhibits melanogenesis in B16 mouse melanoma cells via glycogen synthase kinase 3β phosphorylation. J Pharm Pharmacol. 2011; 63:1031–1036. PMID:
21718286.

20. Lee HE, Kim EH, Choi HR, Sohn UD, Yun HY, Baek KJ, Kwon NS, Park KC, Kim DS. Dipeptides Inhibit Melanin Synthesis in Mel-Ab Cells through Down-Regulation of Tyrosinase. Korean J Physiol Pharmacol. 2012; 16:287–291. PMID:
22915995.

21. Noh JM, Kwak SY, Kim DH, Lee YS. Kojic acid-tripeptide amide as a new tyrosinase inhibitor. Biopolymers. 2007; 88:300–307. PMID:
17211869.

22. Kim EH, Jeong HS, Yun HY, Baek KJ, Kwon NS, Park KC, Kim DS. Geranylgeranylacetone inhibits melanin synthesis via ERK activation in Mel-Ab cells. Life Sci. 2013; 93:226–232. PMID:
23792203.

23. Poumay Y, Coquette A. Modelling the human epidermis in vitro: tools for basic and applied research. Arch Dermatol Res. 2007; 298:361–369. PMID:
17072628.

24. Ponec M, Boelsma E, Weerheim A, Mulder A, Bouwstra J, Mommaas M. Lipid and ultrastructural characterization of reconstructed skin models. Int J Pharm. 2000; 203:211–225. PMID:
10967443.

25. Grabbe J, Welker P, Dippel E, Czarnetzki BM. Stem cell factor, a novel cutaneous growth factor for mast cells and melanocytes. Arch Dermatol Res. 1994; 287:78–84. PMID:
7537033.

26. Yada Y, Higuchi K, Imokawa G. Effects of endothelins on signal transduction and proliferation in human melanocytes. J Biol Chem. 1991; 266:18352–18357. PMID:
1917960.

27. Wang Z, Li X, Yang Z, He X, Tu J, Zhang T. Effects of aloesin on melanogenesis in pigmented skin equivalents. Int J Cosmet Sci. 2008; 30:121–130. PMID:
18377621.

28. Limat A, Salomon D, Carraux P, Saurat JH, Hunziker T. Human melanocytes grown in epidermal equivalents transfer their melanin to follicular outer root sheath keratinocytes. Arch Dermatol Res. 1999; 291:325–332. PMID:
10421058.