Abstract
The aim of the present study was to examine the effect of micellar systems on the absorption of beta-lapachone (b-lap) through different intestinal segments using a single-pass rat intestinal perfusion technique. B-lap was solubilized in mixed micelles composed of phosphatidylcholine and sodium deoxycholate, and in sodium lauryl sulfate (SLS)-based conventional micelles. Both mixed micelles and SLS micelles improved the in situ permeability of b-lap in all intestinal segments tested although the mixed micellar formulation was more effective in increasing the intestinal absorption of b-lap. The permeability of b-lap was greatest in the large intestinal segments. Compared with SLS micelles, the effective permeability coefficient values measured with mixed micelles were 5- to 23-fold higher depending on the intestinal segment. Our data suggest that b-lap should be delivered to the large intestine using a mixed micellar system for improved absorption.
Many drug candidates are poorly soluble in water. In the case of highly hydrophobic drugs, overall absorption process is quite slow due to limited solubility in gastrointestinal fluids and therefore the bioavailability of such drugs is generally low [1-3]. Several approaches have been employed to improve bioavailability including lipid-based formulations, micronization, solid dispersions, and use of colloidal carriers [4-8]. Among these, colloidal carriers such as liposomes, micelles, and nanoparticles are especially promising because they are able to increase both solubility of the drugs and drug transport across the absorptive membranes [9].
Micelles are frequently prepared with pharmaceutically acceptable surfactants and are considered to be one of the most promising absorption-enhancing vehicles because they combine solubilization of drugs within the micelle cores and strong interaction between micellar carriers and absorptive membranes to increase drug bioavailability. Mixed micelles, generally composed of bile salts and phospholipids, have been also been shown to be useful in increasing bioavailability of poorly water-soluble drugs.
Beta-lapachone (b-lap) (Fig. 1) is currently under development for the treatment of obesity and for anti-tumor activities [10,11]. In our laboratory, we estimated the solubility of b-lap to be approximately 35 µg/ml in water, which is regarded as insoluble. In previous studies, the formation of inclusion complexes with cyclodextrins including hydroxypropyl-β-cyclodextrin and methylated-β-cyclodextrin has led to an increase in the water solubility of b-lap and allowed the drug to be successfully administered intraperitoneally [12]. However, to our knowledge it has never been delivered via the oral route. Therefore, we have attempted to develop micelle-based oral delivery systems for b-lap because oral delivery offers numerous advantages over parenteral administration and micellar carriers provide the intrinsic advantages mentioned above [13-15].
To develop an oral formulation of b-lap, we examined the impact of micelle types on the absorption properties of b-lap across the small and large intestine using an in situ single-pass perfusion model that is frequently employed in the evaluation of absorption properties of drugs in the gastrointestinal tract. We also evaluated the effective permeability of b-lap in four different intestinal segments (duodenum, jejunum, ileum, and large intestine) to assess site dependency of absorption. The specific aim of the study was, therefore, to compare the intestinal permeability profiles of b-lap from conventional micelles prepared with SLS and mixed micelles prepared with a mixture of sodium deoxycholate and phosphatidylcholine together with evaluation of regional absorption variability.
B-lap, phosphatidylcholine and sodium deoxycholic acid were purchased from Sigma-Aldrich Company (MO, USA). Sodium lauryl sulfate (SLS) and phenol red were purchased from Duksan Pure Chemical Industries (Seoul, Korea). Ketamine was obtained from Yuhan Corp. (Seoul, Korea). All other reagents were analytical grade and deionized water was used throughout.
Male SPF/VAF outbred rats weighing 240~260 g were obtained from Orient Bio Inc. (Sungnam, Korea). The rats were housed under constant temperature (22±2℃) in the animal facility at the College of Pharmacy, Chung-Ang University and were fed a commercial diet with tap water provided ad libitum. The rats were acclimated for at least 5 days before the experiments and fasted overnight prior to the perfusion study.
The SLS-based micellar formulation (SLS micelles) was prepared by adding b-lap (20 mg) and phenol red (2 mg) as a non-absorbable marker to 100 ml SLS solution (1% SLS in 50 mM Tris-HCl, pH 7.4). This mixture was completely dissolved by constant stirring (500 rpm) on a hot plate for 16 hr.
Mixed micelles were composed of a mixture of phosphatidylcholine and sodium deoxycholate in a molar ratio of 1: 2. Phosphatidylcholine and sodium deoxycholate were solubilized in chloroform:methanol (2:1 v/v) in a clean, dry, round-bottom flask and the solvent was evaporated in a rotary evaporator. The resulting thin film was hydrated with 50 mM Tris-HCl (pH 7.4) followed by sonication for 5 min. The drug was added to mixed micelles in a molar ratio of b-lap:mixed micelle of 1:6 [16] and vortexed for 30 sec. Phenol red (20 µg/ml) was also added to the above solution as a marker.
Colloidal properties of micellar formulations of b-lap such as average size, size distribution, and zeta-potential values were determined by dynamic light scattering method with a Zetasizer Nano-ZS (Malvern Instrument, Worcestershire, UK).
The rats were fasted overnight before the perfusion experiment with free access to tap water only. Rats were anesthetized with an intraperitoneal injection of ketamine (60 mg/kg) and then placed on a pad under a surgical lamp to maintain body temperature. After verification of loss of the pain reflex, a midline abdominal incision was made and each intestinal segment (duodenum, jejunum, ileum, or large intestine) of 10 cm was cannulated at both ends with silicone tubing (internal diameter 2 mm). Both cannulaes were secured with surgical silk sutures. Each intestinal segment was identified as follows: duodenum segment beginning from pylorus, jejunum segment beginning 25 cm from the pylorus, ileum segment beginning 20 cm above caecum, large intestine segment beginning at caecum. Approximately 10 cm of the inlet tubing was placed inside the abdominal cavity to allow the perfusion solution to enter the intestinal segment at body temperature [17]. The segment was rinsed with saline (37℃) until a clear perfusate flowed and drops of saline were added to the surgical area then covered with wet gauze to avoid loss of fluid. The experiment was initiated by rapidly filling the segment with the perfusion solution and the solution was perfused with a peristaltic pump at a rate of 0.4 ml/min. After approximately 20 min, when steady-state was achieved, the outlet perfused samples were collected at 20 min intervals up to 120 min [18,19].
Perfusion solution was filtered with 0.45 µm nylon membrane (Whatman Inc., Florham Park, NJ). Levels of b-lap and phenol red in perfusion samples were analyzed using a Hitachi HPLC system. b-Lap and phenol red were separated on a Capcell Park C18 column (4.6×150 mm, 5 µm, Shiseido, Tokyo) with the mobile phase consisting of methanol : water (60:40 v/v) maintained at 30℃. The flow rate was set at 1.0 ml/min and the eluent was monitored at 254 for b-lap and 430 nm for phenol red, respectively.
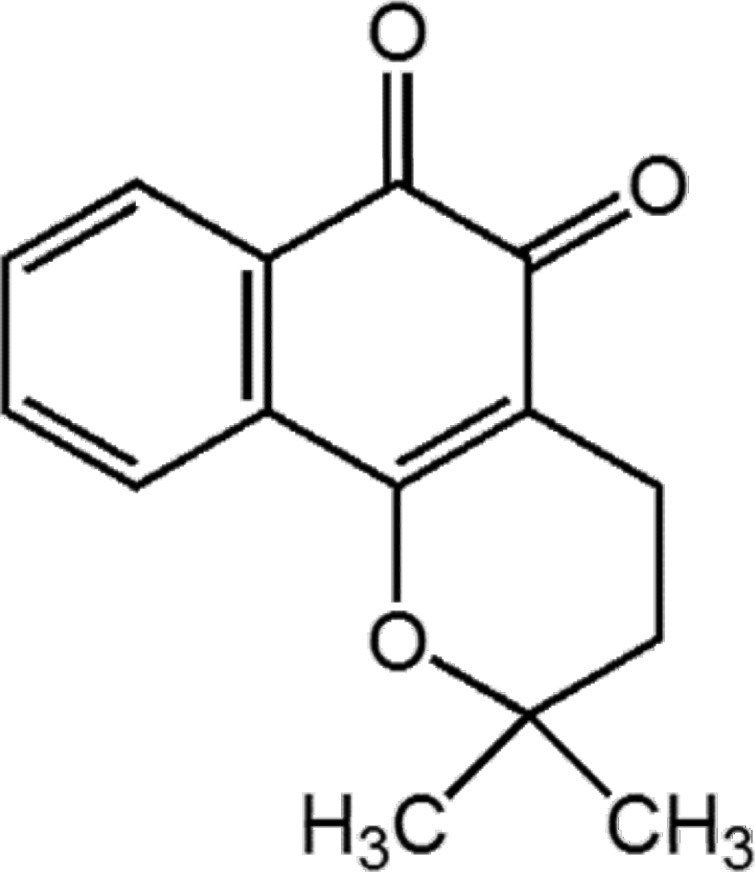
Effective permeability coefficient (Peff) was calculated from the steady-state concentrations achieved after approximately 120 min. The effective permeability at steady-state (Peff, cm/s) was calculated according to a parallel tube model [17]:
Where Qin is the perfusion flow rate (0.4 ml/min); the mass transfer surface area within the intestinal segment was assumed to be the area of a cylinder (2πrl), with a radius (r) of 0.18 cm and length (l) of 10 cm; and Cin and Cout are the respective inlet and fluid-transport-corrected outlet solution concentrations (corrected for water transport). The outlet concentration of b-lap was corrected by multiplying the inlet concentration by [phenol red]in/[phenol red]out determined by HPLC [17]. Data are presented as mean±standard deviation (n=3).
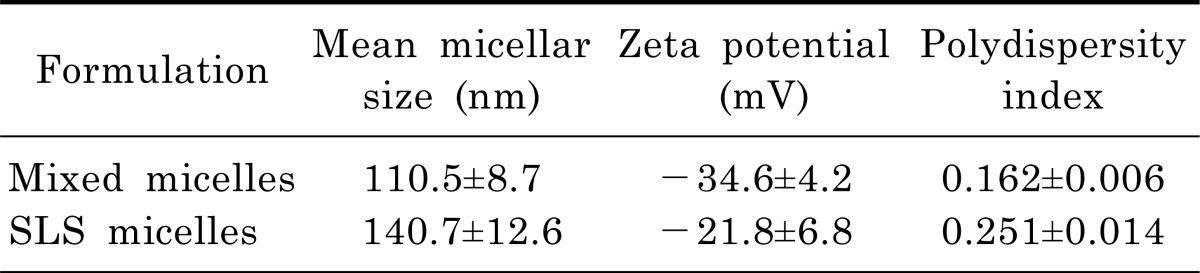
We evaluated physical characteristics of mixed micelles and SLS micelles including mean particle size, polydispersity index, and zeta potential (Table 1). The mean diameter of micellar formulations was 110.5±8.7 nm for mixed micelles and 140.7±12.6 nm for SLS micelles. As a result of a polydispersity index of less than 0.3 for micellar formulations, the particle size distribution was estimated to be narrow. Although the zeta potential values of the mixed micelles were greater than that of SLS micelles, both micellar formulations were regarded as stable when considering the zeta potential values [20].
The single-pass intestinal perfusion method was used to estimate the effective permeability of b-lap in different intestinal segments and compare the intrinsic absorptivity of b-lap formulated into mixed micelles and SLS micelles. Since b-lap is poorly soluble in water, an in situ perfusion study is not feasible without solubilization of the drug. In the present study, micellar formulations were selected as a delivery system for b-lap as they can solubilize b-lap and are expected to enhance absorption of the drug. Moreover, the in situ perfusion study was carried out with different segments of the intestinal tract to evaluate regional variability in absorption.
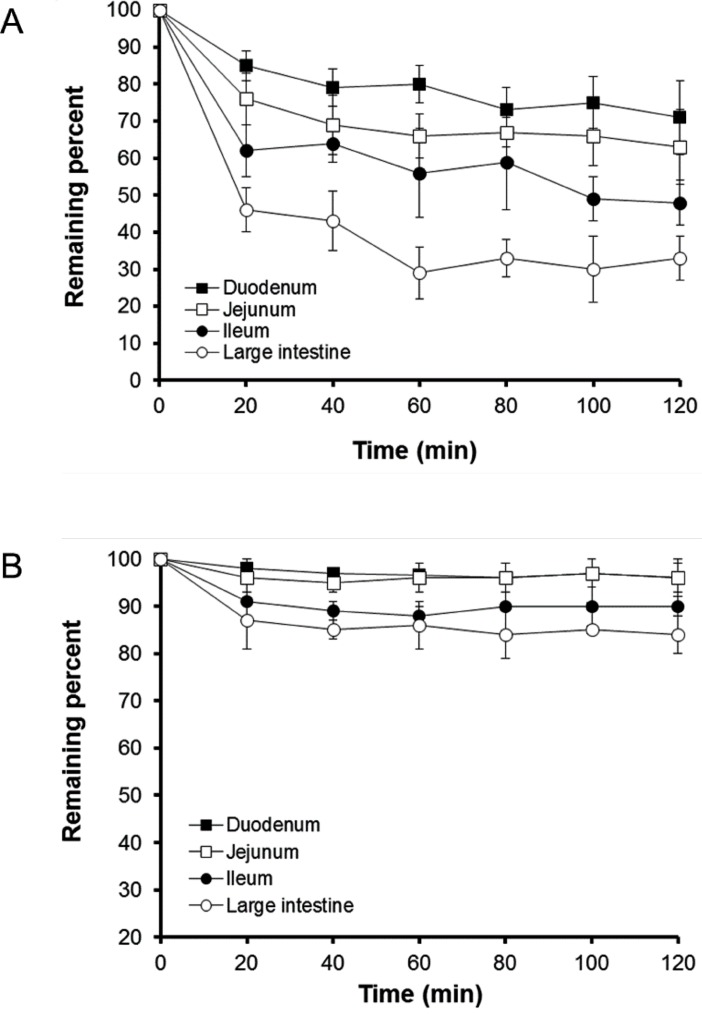
The profiles of percentage of b-lap remaining in mixed micelles and SLS micelles following perfusion through different intestinal segments are shown in Fig. 2. The drug was fairly rapidly absorbed from mixed micelle solutions for the initial 20 min. In particular, drug absorption from the large intestine was the greatest among the intestinal segments tested. Although the rate of b-lap absorption from SLS micelles was slower than that achieved with mixed micelles, drug absorption from SLS micelles was also greatest through the large intestine. Approximately 65% of the initial dose of b-lap from mixed micelles was absorbed through the large intestine over 120 min. The reason for the enhanced absorption of b-lap in micellar formulations through the large intestine may be related to the membrane properties of the intestinal tract. The unstirred water layer located adjacent to the intestinal wall may be a rate-limiting barrier for absorption of lipophilic molecules in the in situ models [21,22]. This layer is known to be much thinner in the large intestine than in other intestinal segments. Another plausible explanation for the best absorption of b-lap at large intestine is that the lipid composition of the colonocytes favors the partitioning of lipophilic components such as phospholipids. The other study revealed that the colon is more permeable to lipophilic molecules than the small intestine [23]. The present study also revealed that micellar formulations might efficiently lower the barrier property of the large intestinal tract. The reason for the greater absorption from mixed micelles compared with SLS micelles remains unclear but we speculate that interaction between micellar components and the intestinal membrane is probably easier for micellar formulations containing a component of the intestinal membrane such as phosphatidylcholine [24]. The reason for the employment of in situ perfusion model for current study was to monitor the difference between the inlet and outlet concentrations that is attributed to absorption from different micellar vehicles. To test this drug was perfectly solubilized in micellar solution (perfusion solution) and therefore the pH effect could be minimized even though the physiological pH of the gut lumen such as duodenum, jejunum, ileum and large intestine is different and varied.
Steady-state concentrations in collected samples can be obtained from in situ absorption profiles in Fig. 2. Steady-state was confirmed by the ratio of outlet to inlet concentrations versus time and was achieved when (Cout/Cin) of b-lap remained constant. For mixed micelles, the ratio of outlet to inlet concentrations decreased significantly, compared to that of SLS micelles as a function of time in all intestinal segments tested. The ratio of outlet to inlet concentrations for mixed and SLS micelles became approximately constant 20 min after the experiment was initiated. The reason for the gradual changes in Cout/Cin values in mixed micelles might be due to the continuing absorption of b-lap. Thus, effective permeability coefficient values were calculated from the steady-state concentrations of b-lap in the perfusate collected from the outlet at 120 min.
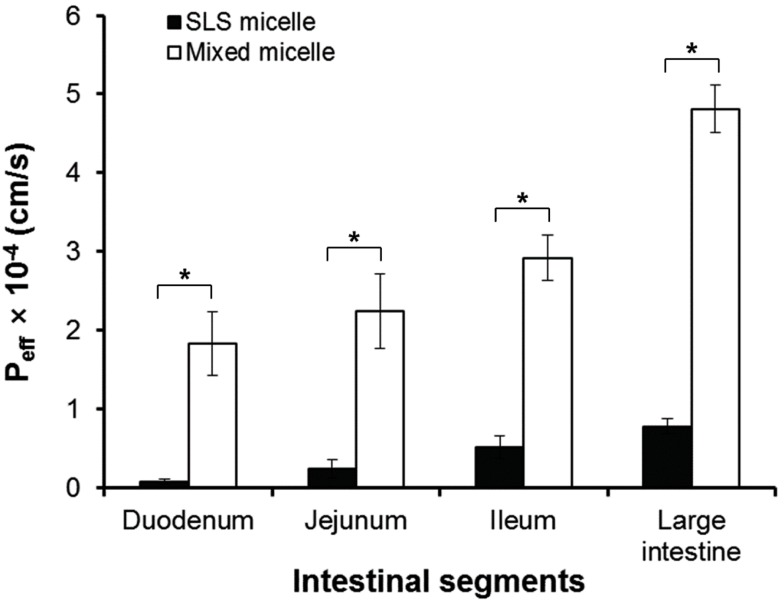
As shown in Fig. 3, the effective permeability values of b-lap in mixed micelles were significantly higher (p<0.05) than those of b-lap in SLS micelles. The estimated effective permeability values of b-lap in mixed micelles at 120 min in the duodenum, jejunum, ileum, and large intestine were 1.83, 2.24, 2.92, and 4.81×10-4 cm/s, respectively. These values were significantly greater than those measured with SLS micelles, which were 0.79, 2.37, 5.15, and 7.82×10-5 cm/s. These results imply that the permeability coefficient values measured with mixed micelles were almost 5- to 23-fold higher than those measured with SLS micelles, depending on the intestinal segment.
Both mixed micelles and SLS micelles improved the in situ permeability of b-lap in all intestinal segments tested. The mixed micellar formulation was more effective in increasing the intestinal absorption of b-lap and the permeability of b-lap was greatest in the large intestinal segments for both formulations. We therefore suggest that b-lap should desirably be delivered to the large intestine with mixed micellar systems for better absorption.
ACKNOWLEDGEMENTS
This research was supported by Chung-Ang University Research Scholarship Grants in 2011.
References
1. Sakuma S, Tanno FK, Masaoka Y, Kataoka M, Kozaki T, Kamaguchi R, Kokubo H, Yamashita S. Effect of administration site in the gastrointestinal tracton bioavailability of poorly absorbed drugstaken after a meal. J Control Release. 2007; 118:59–64. PMID: 17250919.
2. Persson EM, Nordgren A, Forsell P, Knutson L, Ohgren C, Forssén S, Lennernäs H, Abrahamsson B. Improved understanding of the effect of food on drug absorption and bioavailability for lipophilic compounds using an intestinal pig perfusion model. Eur J Pharm Sci. 2008; 34:22–29. PMID: 18387789.

3. Boyd BJ, Khoo SM, Whittaker DV, Davey G, Porter CJ. A lipid-based liquid crystalline matrix that provides sustained release and enhanced oral bioavailability for a model poorly water soluble drug in rats. Int J Pharm. 2007; 340:52–60. PMID: 17467935.

4. Sheen PC, Khetarpal VK, Cariola CM, Rowlings CE. Formulation studies of a poorly water-soluble drug in solid dispersions to improve bioavailability. Int J Pharm. 1995; 118:221–227.

5. Hauss DJ, Fogal SE, Ficorilli JV, Price CA, Roy T, Jayaraj AA, Keirns JJ. Lipid-based delivery systems for improving the bioavailability and lymphatic transport of a poorly water-soluble LTB4 inhibitor. J Pharm Sci. 1998; 87:164–169. PMID: 9519148.

6. McInnes GT, Asbury MJ, Ramsay LE, Shelton JR, Harrison IR. Effect of micronization on the bioavailability and pharmacologic activity of spironolactone. J Clin Pharmacol. 1982; 22:410–417. PMID: 7130430.

7. Mainardes RM, Urban MC, Cinto PO, Khalil NM, Chaud MV, Evangelista RC, Gremiao MP. Colloidal carriers for ophthalmic drug delivery. Curr Drug Targets. 2005; 6:363–371. PMID: 15857294.

8. O'Driscoll CM. Lipid-based formulations for intestinal lymphatic delivery. Eur J Pharm Sci. 2002; 15:405–415. PMID: 12036717.
9. Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther. 2008; 83:761–769. PMID: 17957183.

10. Schiller WR. Summary of proceedings at the 43rd annual pancreas club meeting. J Gastrointest Surg. 2009; 13:2201–2211.

11. Noh JY, Park JS, Lim KM, Kim K, Bae ON, Chung SM, Shin S, Chung JH. A naphthoquinone derivative can induce anemia through phosphatidylserine exposure-mediated erythrophagocytosis. J Pharmacol Exp Ther. 2010; 333:414–420. PMID: 20164298.

12. Cunha-Filho MS, Dacunha-Marinho B, Torres-Labandeira JJ, Martínez-Pacheco R, Landín M. Characterization of betalapachone and methylated beta-cyclodextrin solid-state systems. AAPS PharmSciTech. 2007; 8:E60. PMID: 17915810.
13. Li DX, Han MJ, Balakrishnan P, Yan YD, Oh DH, Joe JH, Seo Y, Kim JO, Park SM, Yong CS, Choi HG. Enhanced oral bioavailability of flurbiprofen by combined use of micelle solution and inclusion compound. Arch Pharm Res. 2010; 33:95–101. PMID: 20191350.

14. Yu JN, Zhu Y, Wang L, Peng M, Tong SS, Cao X, Qiu H, Xu XM. Enhancement of oral bioavailability of the poorly water-soluble drug silybin by sodium cholate/phospholipid-mixed micelles. Acta Pharmacol Sin. 2010; 31:759–764. PMID: 20523347.

15. des Rieux A, Fievez V, Garinot M, Schneider YJ, Préat V. Nanoparticles as potential oral delivery systems of proteins and vaccines: a mechanistic approach. J Control Release. 2006; 116:1–27. PMID: 17050027.

16. Suresh D, Srinivasan K. Studies on the in vitro absorption of spice principles--curcumin, capsaicin and piperine in rat intestines. Food Chem Toxicol. 2007; 45:1437–1442. PMID: 17524539.
17. Song NN, Li QS, Liu CX. Intestinal permeability of metformin using single-pass intestinal perfusion in rats. World J Gastroenterol. 2006; 12:4064–4070. PMID: 16810761.

18. Cook TJ, Shenoy SS. Intestinal permeability of chlorpyrifos using the single-pass intestinal perfusion method in the rat. Toxicology. 2003; 184:125–133. PMID: 12499115.

19. Kunes M, Svoboda Z, Květina J, Herout V, Herink J, Bajgar J. Intestinal single-pass in situ perfusion technique in rat: the influence of L-carnitine on absorption of 7-methoxytacrine. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2005; 149:433–435. PMID: 16601805.
20. Liu J, Gong T, Wang C, Zhong Z, Zhang Z. Solid lipid nanoparticles loaded with insulin by sodium cholate-phosphatidylcholine-based mixed micelles: preparation and characterization. Int J Pharm. 2007; 340:153–162. PMID: 17428627.

21. Levitt MD, Furne JK, Levitt DG. Shaking of the intact rat and intestinal angulation diminish the jejunal unstirred layer. Gastroenterology. 1992; 103:1460–1466. PMID: 1426864.

22. Winne D, Görig H, Müller U. Closed rat jejunal segment in situ: role of pre-epithelial diffusion resistance (unstirred layer) in the absorption process and model analysis. Naunyn Schmiedebergs Arch Pharmacol. 1987; 335:204–215. PMID: 3561532.

23. Lindahl A, Sandström R, Ungell AL, Lennernäs H. Concentration-and region-dependent intestinal permeability of fluvastatin in the rat. J Pharm Pharmacol. 1998; 50:737–744. PMID: 9720622.
24. Hamada T, Ikeda I, Takashima K, Kobayashi M, Kodama Y, Inoue T, Matsuoka R, Imaizumi K. Hydrolysis of micellar phosphatidylcholine accelerates cholesterol absorption in rats and Caco-2 cells. Biosci Biotechnol Biochem. 2005; 69:1726–1732. PMID: 16195591.

Fig. 2
Plots showing remaining percentage of b-lap in mixed micelles (A) and SLS micelles (B) versus time in four intestinal segments of rats using an in situ single-pass perfusion method (Mean±SD, n=3).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


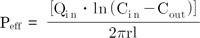
 XML Download
XML Download