1. Lee SH, Kim T, Jeong D, Kim N, Choi Y. The tec family tyrosine kinase Btk Regulates RANKL-induced osteoclast maturation. J Biol Chem. 2008; 283:11526–11534. PMID:
18281276.

2. Miyazaki T, Katagiri H, Kanegae Y, Takayanagi H, Sawada Y, Yamamoto A, Pando MP, Asano T, Verma IM, Oda H, Nakamura K, Tanaka S. Reciprocal role of ERK and NF-kappaB pathways in survival and activation of osteoclasts. J Cell Biol. 2000; 148:333–342. PMID:
10648566.
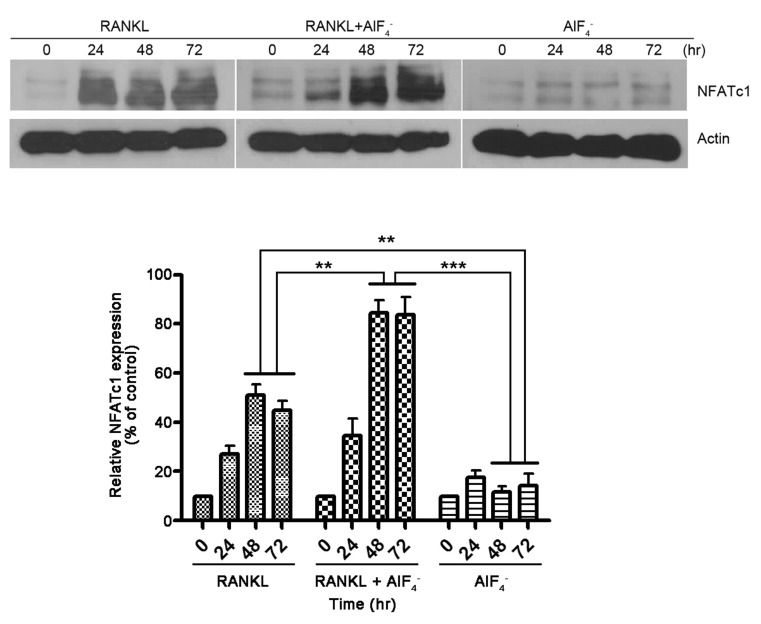
3. Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, Wagner EF, Mak TW, Kodama T, Taniguchi T. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002; 3:889–901. PMID:
12479813.

4. Takayanagi H, Kim S, Matsuo K, Suzuki H, Suzuki T, Sato K, Yokochi T, Oda H, Nakamura K, Ida N, Wagner EF, Taniguchi T. RANKL maintains bone homeostasis through c-Fos-dependent induction of interferon-beta. Nature. 2002; 416:744–749. PMID:
11961557.
5. Yao GQ, Sun Bh, Hammond EE, Spencer EN, Horowitz MC, Insogna KL, Weir EC. The cell-surface form of colony-stimulating factor-1 is regulated by osteotropic agents and supports formation of multinucleated osteoclast-like cells. J Biol Chem. 1998; 273:4119–4128. PMID:
9461606.

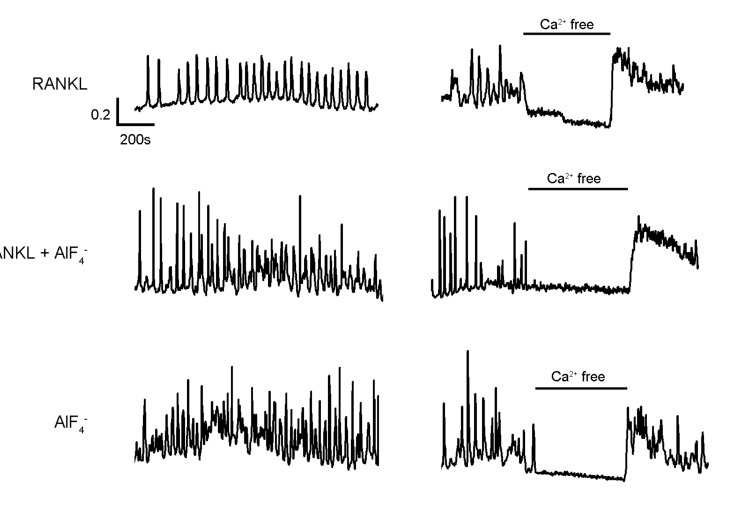
6. Kim MS, Yang YM, Son A, Tian YS, Lee SI, Kang SW, Muallem S, Shin DM. RANKL-mediated reactive oxygen species pathway that induces long lasting Ca
2+ oscillations essential for osteoclastogenesis. J Biol Chem. 2010; 285:6913–6921. PMID:
20048168.
7. Koga T, Inui M, Inoue K, Kim S, Suematsu A, Kobayashi E, Iwata T, Ohnishi H, Matozaki T, Kodama T, Taniguchi T, Takayanagi H, Takai T. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature. 2004; 428:758–763. PMID:
15085135.

8. Pereverzev A, Komarova SV, Korcok J, Armstrong S, Tremblay GB, Dixon SJ, Sims SM. Extracellular acidification enhances osteoclast survival through an NFAT-independent, protein kinase C-dependent pathway. Bone. 2008; 42:150–161. PMID:
17964236.

9. Li H, Wang D, Singh LS, Berk M, Tan H, Zhao Z, Steinmetz R, Kirmani K, Wei G, Xu Y. Abnormalities in osteoclastogenesis and decreased tumorigenesis in mice deficient for ovarian cancer G protein-coupled receptor 1. PLoS One. 2009; 4:e5705. PMID:
19479052.

10. Kato K, Morita I. Promotion of osteoclast differentiation and activation in spite of impeded osteoblast-lineage differentiation under acidosis: effects of acidosis on bone metabolism. Biosci Trends. 2013; 7:33–41. PMID:
23524891.

11. Iwai K, Koike M, Ohshima S, Miyatake K, Uchiyama Y, Saeki Y, Ishii M. RGS18 acts as a negative regulator of osteoclastogenesis by modulating the acid-sensing OGR1/NFAT signaling pathway. J Bone Miner Res. 2007; 22:1612–1620. PMID:
17576169.

12. Yang M, Mailhot G, Birnbaum MJ, MacKay CA, Mason-Savas A, Odgren PR. Expression of and role for ovarian cancer G-protein-coupled receptor 1 (OGR1) during osteoclastogenesis. J Biol Chem. 2006; 281:23598–23605. PMID:
16787916.

13. Strunecká A, Strunecký O, Patocka J. Fluoride plus aluminum: useful tools in laboratory investigations, but messengers of false information. Physiol Res. 2002; 51:557–564. PMID:
12511178.
14. Li L. The biochemistry and physiology of metallic fluoride: action, mechanism, and implications. Crit Rev Oral Biol Med. 2003; 14:100–114. PMID:
12764073.

15. Sui G, Fry CH, Malone-Lee J, Wu C. Aberrant Ca
2+ oscillations in smooth muscle cells from overactive human bladders. Cell Calcium. 2009; 45:456–464. PMID:
19345414.
16. Chong SA, Hong SY, Moon SJ, Park JW, Hong JH, An JM, Lee SI, Shin DM, Seo JT. Partial inhibition of SERCA is responsible for extracellular Ca
2+ dependence of AlF-4-induced [Ca
2+]
i oscillations in rat pancreatic. Am J Physiol Cell Physiol. 2003; 285:C1142–C1149. PMID:
12878491.
17. Lau KH, Yoo A, Wang SP. Aluminum stimulates the proliferation and differentiation of osteoblasts in vitro by a mechanism that is different from fluoride. Mol Cell Biochem. 1991; 105:93–105. PMID:
1922012.

18. Zaidi M, Datta HK, Moonga BS, MacIntyre I. Evidence that the action of calcitonin on rat osteoclasts is mediated by two G proteins acting via separate post-receptor pathways. J Endocrinol. 1990; 126:473–481. PMID:
2170558.

19. Yang YM, Jung HH, Lee SJ, Choi HJ, Kim MS, Shin DM. TRPM7 Is Essential for RANKL-Induced Osteoclastogenesis. Korean J Physiol Pharmacol. 2013; 17:65–71. PMID:
23440520.

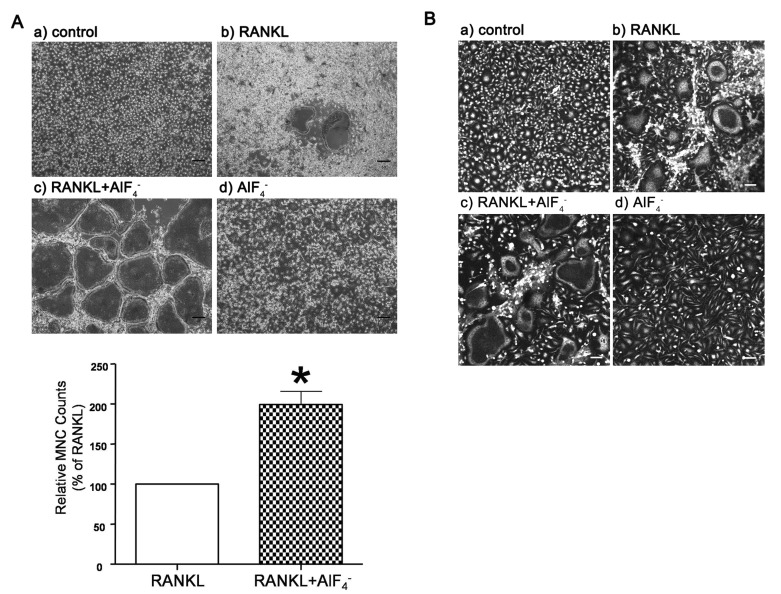
20. Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 1998; 95:3597–3602. PMID:
9520411.

21. Sharma SM, Bronisz A, Hu R, Patel K, Mansky KC, Sif S, Ostrowski MC. MITF and PU.1 recruit p38 MAPK and NFATc1 to target genes during osteoclast differentiation. J Biol Chem. 2007; 282:15921–15929. PMID:
17403683.

22. Sato K, Suematsu A, Nakashima T, Takemoto-Kimura S, Aoki K, Morishita Y, Asahara H, Ohya K, Yamaguchi A, Takai T, Kodama T, Chatila TA, Bito H, Takayanagi H. Regulation of osteoclast differentiation and function by the CaMK-CREB pathway. Nat Med. 2006; 12:1410–1416. PMID:
17128269.

23. Feng H, Cheng T, Steer JH, Joyce DA, Pavlos NJ, Leong C, Kular J, Liu J, Feng X, Zheng MH, Xu J. Myocyte enhancer factor 2 and microphthalmia-associated transcription factor cooperate with NFATc1 to transactivate the V-ATPase d2 promoter during RANKL-induced osteoclastogenesis. J Biol Chem. 2009; 284:14667–14676. PMID:
19321441.

24. Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003; 4:517–529. PMID:
12838335.

25. Son A, Kim MS, Jo H, Byun HM, Shin DM. Effects of inositol 1,4,5-triphosphate on osteoclast differentiation in RANKL-induced osteoclastogenesis. Korean J Physiol Pharmacol. 2012; 16:31–36. PMID:
22416217.

26. Yang S, Li YP. RGS10-null mutation impairs osteoclast differentiation resulting from the loss of [Ca
2+]
i oscillation regulation. Genes Dev. 2007; 21:1803–1816. PMID:
17626792.
27. Sternweis PC, Northup JK, Smigel MD, Gilman AG. The regulatory component of adenylate cyclase. Purification and properties. J Biol Chem. 1981; 256:11517–11526. PMID:
6271754.

28. Carter RH, Park DJ, Rhee SG, Fearon DT. Tyrosine phosphorylation of phospholipase C induced by membrane immunoglobulin in B lymphocytes. Proc Natl Acad Sci USA. 1991; 88:2745–2749. PMID:
2011584.

29. Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000; 1:11–21. PMID:
11413485.

30. Neves SR, Ram PT, Iyengar R. G protein pathways. Science. 2002; 296:1636–1639. PMID:
12040175.

31. Tomura H, Wang JQ, Liu JP, Komachi M, Damirin A, Mogi C, Tobo M, Nochi H, Tamoto K, Im DS, Sato K, Okajima F. Cyclooxygenase-2 expression and prostaglandin E2 production in response to acidic pH through OGR1 in a human osteoblastic cell line. J Bone Miner Res. 2008; 23:1129–1139. PMID:
18302504.

32. Hanami K, Nakano K, Saito K, Okada Y, Yamaoka K, Kubo S, Kondo M, Tanaka Y. Dopamine D2-like receptor signaling suppresses human osteoclastogenesis. Bone. 2013; 56:1–8. PMID:
23631878.

33. Fisch TM, Prywes R, Simon MC, Roeder RG. Multiple sequence elements in the c-fos promoter mediate induction by cAMP. Genes Dev. 1989; 3:198–211. PMID:
2541049.

34. Lee SH, Rho J, Jeong D, Sul JY, Kim T, Kim N, Kang JS, Miyamoto T, Suda T, Lee SK, Pignolo RJ, Koczon-Jaremko B, Lorenzo J, Choi Y. v-ATPase V0 subunit d2-deficient mice exhibit impaired osteoclast fusion and increased bone formation. Nat Med. 2006; 12:1403–1409. PMID:
17128270.

35. Tanaka S, Nakamura I, Inoue J, Oda H, Nakamura K. Signal transduction pathways regulating osteoclast differentiation and function. J Bone Miner Metab. 2003; 21:123–133. PMID:
12720046.

36. Leibbrandt A, Penninger JM. RANK/RANKL: regulators of immune responses and bone physiology. Ann N Y Acad Sci. 2008; 1143:123–150. PMID:
19076348.