Abstract
Here, we show that radicicol, a fungal antibiotic, resulted in marked inhibition of inducible nitric oxide synthase (iNOS) transcription by the pancreatic beta cell line MIN6N8a in response to cytokine mixture (CM: TNF-α, IFN-γ, and IL-1β). Treatment of MIN6N8a cells with radicicol inhibited CM-stimulated activation of NF-κB/Rel, which plays a critical role in iNOS transcription, in a dose-related manner. Nitrite production in the presence of PD98059, a specific inhibitor of the extracellular signal-regulated protein kinase-1 and 2 (ERK1/2) pathway, was dramatically diminished, suggesting that the ERK1/2 pathway is involved in CM-induced iNOS expression. In contrast, SB203580, a specific inhibitor of p38, had no effect on nitrite generation. Collectively, this series of experiments indicates that radicicol inhibits iNOS gene expression by blocking ERK1/2 signaling. Due to the critical role that NO release plays in mediating destruction of pancreatic beta cells, the inhibitory effects of radicicol on iNOS expression suggest that radicicol may represent a useful anti-diabetic activity.
Insulin-dependent Diabetes mellitus (IDDM) is an autoimmune disease that selectively destroys insulin-producing beta cells in the pancreatic islets of Langerhans. Strong experimental evidence suggests that nitric oxide (NO) is produced by proinflammatory cytokines, such as tumor necrosis factor (TNF)-α, interferon (IFN)-γ, and interleukin (IL)-1β and plays an important role in the development of pancreatic beta cell destruction by stimulating production of oxygen radicals [1-3]. Further, IFN-γ, TNF-α and IL-1β synergistically activate inducible NO synthase (iNOS) mRNA expression and NO production [1,4]. Studies on transgenic mice have shown that NO is important in beta cell destruction [5,6]. Specifically, transgenic mice expressing mouse iNOS cDNA under the control of the insulin promoter have been shown to develop diabetes, whereas treatment with aminoguanidine, an iNOS inhibitor, was shown to prevent or delay the development of diabetes [5]. Further, it was shown that IL-1β fails to induce iNOS mRNA expression or increase nitrite formation in islets isolated from iNOS knockout mice [6]. iNOS knockout mice further display reduced sensitivity to multiple low-dose streptozotocin-induced diabetes [6]. Therefore, inhibition of high-output NO production by blocking iNOS production or activity may be a useful strategy for the treatment of inflammatory disorders, including IDDM.
Radicicol is a macrocyclic fungal antibiotic originally isolated from the fungus Monosporium bonorden and inhibits angiogenesis [7,8]. Radicicol induces reversal of the transformed phenotype of src-transformed cells and exhibits p60v-src tyrosine kinase inhibitor activity [9,10]. Radicicol also blocks the activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) pathway through destabilization of Raf kinase [11]. It has been reported that radicicol suppresses the expression of mitogen-inducible cyclooxygenase, which is important in inflammatory responses [12]. We have shown that expression of iNOS, another important mediator of inflammatory responses, p38 kinase pathway activation, and NF-κB activation were inhibited by radicicol in LPS-stimulated macrophages [13]. In the present study, we investigated the effects of radicicol on the regulation of iNOS, NF-κB activity, p65 nuclear translocation, and p44/42 activity in proinflammatory cytokine-stimulated MIN6N8a cells, a mouse pancreatic beta cell line.
MIN6N8a cells, SV40 T-transformed insulinoma cells derived from NOD mice, were grown in Dulbecco's Modifed Eagle's Medium supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 100 U/ml of penicillin, 100 µg/ml of streptomycin, and 50 µM 2-mercaptoethanol. For each experiment, cells (5×105 cells/ml) were plated in 100-mm dishes. Radicicol, SN50, PD98059 (2'-amino-3'-methoxyflavone), and SB203580 (4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(4-pyridyl) 1H-imidazole) were purchased from CalBiochem (San Diego, CA). Anti-iNOS and anti-p65 antibodies were purchased from Upstate Biotechnology (Lake Placid, NY) and Santa Cruz Biotechnology Inc (Santa Cruz, CA), respectively. Antibodies against phospho-p44/42 and p44/42 were purchased from Cell Signaling Technology, Inc. (Beverly, MA).
MIN6N8a cells were treated with the indicated concentrations of radicicol in the presence of cytokine mixture (CM: TNF-α, 500 U/ml; IFN-γ, 100 U/ml; IL-1β, 10 U/ml) for 48 h. Culture supernatants were collected, and the accumulation of NO2- in culture supernatants was measured as an indicator of NO production in the medium as previously described [14,15].
MIN6N8a cells were treated with radicicol (100 ng/ml) in the presence of CM on a cover slide in 12-well plates. Cells were rinsed 3 times with PBS, fixed with 4% paraformaldehyde for 10 min at room temperature, and rinsed again. Cells were then blocked with 1% bovine serum albumin, followed by the addition of the primary antibody. After extensive washing with Tris-buffered saline, fluorescein isothiocyanate-conjugated IgG was added. Following incubation, the slides were rinsed, mounted, and viewed at 488 nm on a confocal microscope (FV300, Olympus, Japan).
Total RNA was isolated with TRI Reagent (Molecular Research Center, Cincinnati, OH, USA). Forward and reverse primer sequences were as follows: iNOS: 5'-CTG CAG CAC TTG GAT CAG GAA CCT G-3', 5'-GGG AGT AGC CTG TGT GCA CCT GGA A-3'; and β-actin: 5'-TGG AAT CCT GTG GCA TCC ATG AAA C-3', 5'-TAA AAC GCA GCT CAG TAA CAG TCC G-3'. Equal amounts of RNA were reverse-transcribed into cDNA with oligo(dT)15 primers. PCR was performed with cDNA and each primer. Samples were heated to 94℃ for 5 min and cycled 30 times at 94℃ for 1 min, 55℃ for 1.5 min, and 94℃ for 1 min, after which an additional extension step at 72℃ for 5 min was conducted. PCR products were separated by 8% SDS-PAGE, followed by staining in ethidium bromide. The iNOS and β-actin primers produced amplified products of 311 and 349 bp, respectively.
Whole cell lysates were separated by 10% SDS-PAGE and then electrotransferred to nitrocellulose membranes (Amersham International, Buckinghamshire, UK). The membranes were then preincubated for 1 h at room temperature in Tris-buffered saline (TBS), pH 7.6 containing 0.05% Tween-20 and 3% bovine serum albumin, followed by incubation with iNOS, phosphorylated ERK1/2, and phospho-ERK1/2 (Thr202/Tyr204)-specific antibodies. Immunoreactive bands were detected by incubation with conjugates of anti-rabbit IgG with horseradish peroxidase and enhanced chemiluminescence reagent (Amersham).
EMSA was performed as described in previous literature [16,17]. Nuclear extracts were prepared as previously described [18]. The double-stranded oligonucleotides were end-labeled with [γ-32P]-ATP. Nuclear extracts (5 µg) were incubated with poly (dI-dC) and the [32P]-labeled DNA probe in binding buffer (100 mM KCl, 30 mM HEPES, 1.5 mM MgCl2, 0.3 mM EDTA, 10% glycerol, 1 mM DTT, 1 mM PMSF, 1 µg/ml of aprotinin, and 1 µg/ml of leupeptin) for 10 min. DNA-binding activity was separated from free probe by 4% SDS-PAGE in 0.5× TBE buffer. Following electrophoresis, the gel was dried and subjected to autoradiography.
Vector constructions were performed as previously described [19]. MIN6N8aMIN6N8a cells were transfected using the DEAE-dextran method, diluted to 5×105 cells per 1 ml of complete media, plated on 24-well plates, and then incubated in the presence of 5% CO2 at 37℃ for 24 h. The transfectants were then treated with CM and radicicol for 18 h, after which cells were lysed with lysis buffer. The lysates were centrifuged (12,000×g for 10 min at 4℃), and the supernatant was assayed for CAT enzyme expression using a CAT ELISA kit (Roche Molecular Biochemicals, Mannheim, Germany) according to the manufacturer's instructions.
The mean±SD was determined for each treatment group in a given experiment. When significant differences occurred, treatment groups were compared to the vehicle controls using a Dunnett's two-tailed t test [20].
Cytokines, including IL-1β, IFN-γ, and TNF-α are known to induce or potentiate iNOS expression and NO production [1-3]. We investigated the effects of radicicol on NO production in cytokine-stimulated MIN6N8a, a mouse pancreatic beta cell line. Cytokine mixture (CM: TNF-α, 500 U/ml; IFN-γ, 100 U/ml; IL-1β, 10 U/ml) increased production of nitrite ≥15-fold over basal levels in MIN6N8a cells (Fig. 1A). This induction of nitrite generation by CM was inhibited by radicicol in a dose-dependent manner. No effect on cell viability was observed in any of the treatment groups, and it always exceeded 90% as determined by trypan blue staining (Fig. 1B).
To investigate the effects of radicicol on iNOS production, expression of iNOS was measured by immunohistochemical staining. MIN6N8a cells (5×105 cells/ml) were incubated with radicicol (100 ng/ml) in the presence of CM for 24 h on a cover slide in 12-well plates. Cells were then subjected to immunohistochemical staining using an antibody specific for murine iNOS. Immunofluorescence staining of iNOS showed that radicicol inhibited iNOS production (Fig. 2A). After MIN6N8a cells were exposed to radicicol in the presence of CM, the expression level of iNOS was monitored by Western immunoblot analysis. As shown in Fig. 2B, iNOS protein production was inhibited by radicicol treatment. Consistent with these findings, radicicol inhibited the production of iNOS mRNA in CM-stimulated MIN6N8a cells (Fig. 2C). Control β-actin was constitutively expressed and was not affected by radicicol treatment. These results indicate that radicicol reduces gene expression of iNOS, which is involved in pancreatic beta cell destruction.
Since it has been reported that protein binding at NF-κB/Rel-binding sequences is necessary for induction of iNOS expression [21], we assessed the effect of radicicol on NF-κB/Rel activation using a transient transfection assay. MIN-6N8a cells were transiently transfected with p(NF-κB/Rel)3-CAT showed reduced gene expression of CAT in response to radicicol treatment in the presence of CM (Fig. 3A). Although CM treatment significantly increased CAT expression by MIN6N8a cells, CM-induced CAT expression was inhibited by radicicol treatment. Oct, a transcription factor that binds to the promoter of the iNOS gene, moderated CAT activity and was not influenced by either CM or radicicol treatment (Fig. 3B). Transcriptional activation of the NF-κB/Rel transcription factor is preceded by its binding to DNA. Therefore, we further analyzed the effect of radicicol on activation of NF-κB/Rel, whose binding motif is in the promoter of the iNOS gene, using EMSA. MIN6N8a cells treated with CM displayed a marked increase in NF-κB/Rel binding to its cognate site. Further, induction of NF-κB/Rel binding was inhibited by radicicol (Fig. 3C). Radicicol had no effect on Oct DNA-binding activity (Fig. 3D). The specificity of the retarded bands was confirmed by the addition of excess 32P-unlabeled double-stranded κB or Oct (data not shown). These results indicate that radicicol reduces the DNA-binding activity and transcriptional activation of NF-κB/Rel, which is important in the regulation of iNOS gene expression.
To further investigate whether or not radicicol inhibits nuclear translocation of p65, a component of NF-κB/Rel, we performed immunofluorescence staining. CM-stimulated MIN6N8a cells showed marked p65 staining in nuclei, whereas unstimulated cells showed weaker nuclear p65 expression along with stronger staining in the cytoplasm. Radicicol treatment significantly inhibited CM-induced nuclear translocation of p65 (Fig. 4A). These results indicate that radicicol reduces the nuclear translocation and DNA-binding activity of NF-κB/Rel, which is important in the regulation of iNOS gene expression.
We further confirmed the involvement of p65 nuclear translocation and NO production using SN50, a NF-κB/Rel nuclear translocation inhibitor. SN50, a cell-permeable inhibitor peptide, was reported to inhibit nuclear translocation of NF-κB/Rel [22]. Treatment of MIN6N8a cells with SN50 blocked CM-induced nitrite generation (Fig. 4B). Collectively, these results demonstrate that NF-κB/Rel plays an important role in the activation of iNOS gene expression by CM in pancreatic beta cells, and it is inhibited by radicicol.
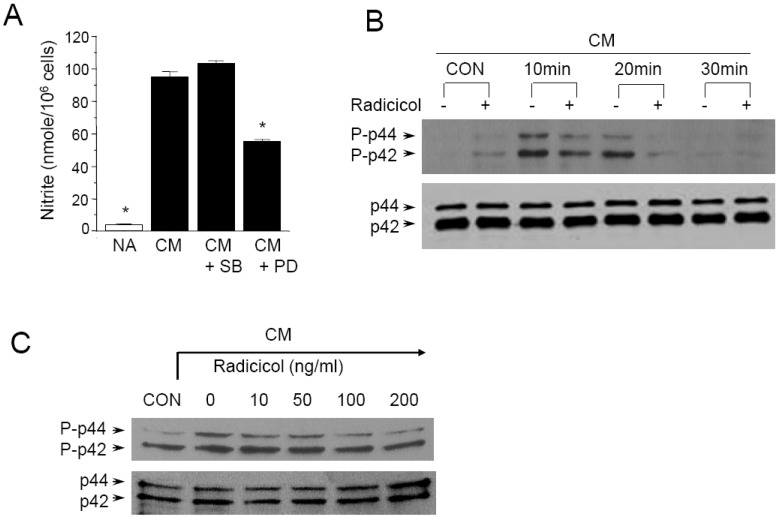
Since p44/42 and p38 kinases are possible targets of radicicol, we further determined whether or not these kinase pathways are involved in CM-induced NO production. The p44/42 and p38 kinase pathways were specifically blocked when MIN6N8a cells were challenged with CM. PD98059 is a specific inhibitor of mitogen activated protein kinase/extracellular signal-regulated kinase 1 (MEK-1), which is responsible for ERK1/2 activation. SB203580, a bicyclic imidazole compound, is a specific inhibitor of p38. PD98059 inhibited CM-induced production of nitrite, whereas SB20-3580P had no inhibitory effect (Fig. 5A). These results suggest that the p44/42 kinase pathway is important in the regulation of NO production in MIN6N8a cells by CM.
We further assessed the effect of radicicol on p44/42 phosphorylation in CM-stimulated MIN6N8a cells. CM-induced phosphorylation of p44/42 peaked at 10 min, was maintained until 20 min, and then decreased at 30 min (Fig. 5B). On the other hand, radicicol strongly inhibited p44/42 phosphorylation. A dose-response experiment further showed that radicicol inhibited CM-induced p44/42 phosphorylation in a dose-dependent manner (Fig. 5C). Collectively, this series of experiments indicates that p44/42 kinases are important in the regulation of iNOS in CM-stimulated MIN6N8a cells, and they are inhibited by radicicol.
In a previous study, we showed that production of NO, an important mediator of inflammatory responses, is inhibited by radicicol in LPS-stimulated macrophages [13]. Since proinflammatory cytokines are known to induce the expression of iNOS mRNA and production of NO, resulting in cell death of beta cells [1-3], we investigated whether or not radicicol suppresses iNOS gene expression induced by cytokines. In the present study, we found that radicicol inhibits iNOS gene expression in cytokine-stimulated MIN6N8a beta cells.
NF-κB/Rel plays an important role in cytokine-induced beta cell destruction, as cell death is blocked in beta cells by inhibition of NF-κB/Rel activation [23,24]. Conditional and specific blockage of NF-κB/Rel protects pancreatic beta cells against diabetes induced by multiple low doses of streptozotocin [25]. We observed that NF-κB/Rel was positively regulated by CM for induction of iNOS gene expression, whereas radicicol significantly inhibited the CM-induced NF-κB/Rel transcriptional activation and DNA-binding activity (Fig. 3). NF-κB/Rel is a regulator of many genes involved in inflammatory responses, including iNOS [21]. NF-κB/Rel exists in the cytoplasm of unstimulated cells in an inactive form bound to IκB, an inhibitor of NF-κB/Rel. Activation by external stimuli including lipopolysaccharide and cytokines results in the phosphorylation of IκB, which releases the active DNA-binding form of NF-κB/Rel for translocation to the nucleus to bind κB motifs in the promoter regions of various genes. EMSA studies showed strong induction of two separate κB-binding complexes by CM. Further, radicicol inhibited activation of both of the binding complexes (Fig. 3C). It has been shown that p50 proteins have DNA-binding activity, whereas p65 proteins contain transactivation domains in their C termini and are thus able to activate the transcription of target genes [26]. Immunofluorescence staining of p65, the active component of NF-κB/Rel, confirmed nuclear translocation of p65 induced by CM, whereas radicicol inhibited nuclear translocation of p65 (Fig. 4A).
We also showed that radicicol significantly inhibits the ERK1/2 pathway in MIN6N8a cells. It has been reported that radicicol blocks the activation of the MAPK/ERK pathway through destabilization of Raf kinase [11,27]. ERK activity is required for iNOS gene expression in insulin-producing INS-1E cells [28]. In the present study, we investigated the involvement of the ERK1/2 and p38 pathways in iNOS regulation in a mouse insulinoma cell line stimulated with cytokines. Two inhibitors, PD98059 and SB203580, which selectively inhibit the ERK1/2 and p38 pathways, respectively, were used to address this issue. Data presented herein clearly show that the ERK1/2 pathway is involved in iNOS regulation in MIN6N8a cells. Specifically, PD98059 inhibited NO production in response to CM. ERK has been reported to be involved in NF-κB/Rel-mediated transcription of iNOS, indicating that ERK regulates iNOS gene expression by increasing the transactivation capacity of NF-κB/Rel [28].
In summary, these experiments demonstrate that radicicol inhibits CM-induced iNOS gene expression in MIN6N8a cells. Based on our findings, the most likely mechanism that can account for this biological effect involves inhibition of NF-κB and the ERK1/2 kinase pathway. Due to the critical role that NO release plays in mediating inflammatory responses in pancreatic beta cells, the inhibitory effects of radicicol on iNOS expression suggest that this anti-fungal may represent a useful anti-diabetic agent.
References
1. Cetkovic-Cvrlje M, Eizirik DL. TNF-alpha and IFN-gamma potentiate the deleterious effects of IL-1 beta on mouse pancreatic islets mainly via generation of nitric oxide. Cytokine. 1994; 6:399–406. PMID: 7948748.
2. Darville MI, Eizirik DL. Regulation by cytokines of the inducible nitric oxide synthase promoter in insulin-producing cells. Diabetologia. 1998; 41:1101–1108. PMID: 9754830.

3. Eizirik DL, Sandler S, Welsh N, Cetkovic-Cvrlje M, Nieman A, Geller DA, Pipeleers DG, Bendtzen K, Hellerström C. Cytokines suppress human islet function irrespective of their effects on nitric oxide generation. J Clin Invest. 1994; 93:1968–1974. PMID: 7514190.

4. Yamada K, Otabe S, Inada C, Takane N, Nonaka K. Nitric oxide and nitric oxide synthase mRNA induction in mouse islet cells by interferon-gamma plus tumor necrosis factor-alpha. Biochem Biophys Res Commun. 1993; 197:22–27. PMID: 7504484.
5. Takamura T, Kato I, Kimura N, Nakazawa T, Yonekura H, Takasawa S, Okamoto H. Transgenic mice overexpressing type 2 nitric-oxide synthase in pancreatic beta cells develop insulin-dependent diabetes without insulitis. J Biol Chem. 1998; 273:2493–2496. PMID: 9446547.
6. Flodström M, Tyrberg B, Eizirik DL, Sandler S. Reduced sensitivity of inducible nitric oxide synthase-deficient mice to multiple low-dose streptozotocin-induced diabetes. Diabetes. 1999; 48:706–713. PMID: 10102685.

7. Fukuhara Y, Turner RJ. Cation dependence of renal outer cortical brush border membrane L-glutamate transport. Am J Physiol. 1985; 248:F869–F875. PMID: 2860810.

8. Oikawa T, Ito H, Ashino H, Toi M, Tominaga T, Morita I, Murota S. Radicicol, a microbial cell differentiation modulator, inhibits in vivo angiogenesis. Eur J Pharmacol. 1993; 241:221–227. PMID: 7694864.
9. Kwon HJ, Yoshida M, Abe K, Horinouchi S, Beppu T. Radicicol, an agent inducing the reversal of transformed phenotypes of src-transformed fibroblasts. Biosci Biotechnol Biochem. 1992; 56:538–539. PMID: 1368339.
10. Kwon HJ, Yoshida M, Fukui Y, Horinouchi S, Beppu T. Potent and specific inhibition of p60v-src protein kinase both in vivo and in vitro by radicicol. Cancer Res. 1992; 52:6926–6930. PMID: 1458481.
11. Soga S, Kozawa T, Narumi H, Akinaga S, Irie K, Matsumoto K, Sharma SV, Nakano H, Mizukami T, Hara M. Radicicol leads to selective depletion of Raf kinase and disrupts K-Ras-activated aberrant signaling pathway. J Biol Chem. 1998; 273:822–828. PMID: 9422737.

12. Chanmugam P, Feng L, Liou S, Jang BC, Boudreau M, Yu G, Lee JH, Kwon HJ, Beppu T, Yoshida M, Xia Y, Wilson CB, Hwand D. Radicicol, a protein tyrosine kinase inhibitor, suppresses the expression of mitogen-inducible cyclooxygenase in macrophages stimulated with lipopolysaccharide and in experimental glomerulonephritis. J Biol Chem. 1995; 270:5418–5426. PMID: 7890656.

13. Jeon YJ, Kim YK, Lee M, Park SM, Han SB, Kim HM. Radicicol suppresses expression of inducible nitric-oxide synthase by blocking p38 kinase and nuclear factor-kappaB/Rel in lipopolysaccharide-stimulated macrophages. J Pharmacol Exp Ther. 2000; 294:548–554. PMID: 10900231.
14. Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem. 1982; 126:131–138. PMID: 7181105.
15. Huong PT, Lee MY, Lee KY, Chang IY, Lee SK, Yoon SP, Lee DC, Jeon YJ. Synergistic induction of iNOS by IFN-γ and glycoprotein isolated from dioscorea batatas. Korean J Physiol Pharmacol. 2012; 16:431–436. PMID: 23269906.
16. Jeon YJ, Yang KH, Pulaski JT, Kaminski NE. Attenuation of inducible nitric oxide synthase gene expression by delta 9-tetrahydrocannabinol is mediated through the inhibition of nuclear factor-kappa B/Rel activation. Mol Pharmacol. 1996; 50:334–341. PMID: 8700141.
17. Li MH, Kothandan G, Cho SJ, Huong PT, Nan YH, Lee KY, Shin SY, Yea SS, Jeon YJ. Magnolol inhibits LPS-induced NF-κB/Rel activation by blocking p38 kinase in murine macrophages. Korean J Physiol Pharmacol. 2010; 14:353–358. PMID: 21311674.

18. Xie QW, Whisnant R, Nathan C. Promoter of the mouse gene encoding calcium-independent nitric oxide synthase confers inducibility by interferon gamma and bacterial lipopolysaccharide. J Exp Med. 1993; 177:1779–1784. PMID: 7684434.

19. Jeon YJ, Han SH, Lee YW, Yea SS, Yang KH. Inhibition of NF-kappa B/Rel nuclear translocation by dexamethasone: mechanism for the inhibition of iNOS gene expression. Biochem Mol Biol Int. 1998; 45:435–441. PMID: 9679644.
20. Dunnett M. A multiple comparison procedure for comparing several treatments with a control. J Am Statistics Assoc. 1955; 50:1096–1121.

21. Xie QW, Kashiwabara Y, Nathan C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J Biol Chem. 1994; 269:4705–4708. PMID: 7508926.

22. Lin YZ, Yao SY, Veach RA, Torgerson TR, Hawiger J. Inhibition of nuclear translocation of transcription factor NF-kappa B by a synthetic peptide containing a cell membrane-permeable motif and nuclear localization sequence. J Biol Chem. 1995; 270:14255–14258. PMID: 7782278.
23. Heimberg H, Heremans Y, Jobin C, Leemans R, Cardozo AK, Darville M, Eizirik DL. Inhibition of cytokine-induced NF-kappaB activation by adenovirus-mediated expression of a NF-kappaB super-repressor prevents beta-cell apoptosis. Diabetes. 2001; 50:2219–2224. PMID: 11574401.
24. Baker MS, Chen X, Cao XC, Kaufman DB. Expression of a dominant negative inhibitor of NF-kappaB protects MIN6 beta-cells from cytokine-induced apoptosis. J Surg Res. 2001; 97:117–122. PMID: 11341786.
25. Eldor R, Yeffet A, Baum K, Doviner V, Amar D, Ben-Neriah Y, Christofori G, Peled A, Carel JC, Boitard C, Klein T, Serup P, Eizirik DL, Melloul D. Conditional and specific NF-kappaB blockade protects pancreatic beta cells from diabetogenic agents. Proc Natl Acad Sci USA. 2006; 103:5072–5077. PMID: 16551748.
26. Schmitz ML, Baeuerle PA. The p65 subunit is responsible for the strong transcription activating potential of NF-kappa B. EMBO J. 1991; 10:3805–3817. PMID: 1935902.

27. Roe SM, Prodromou C, O'Brien R, Ladbury JE, Piper PW, Pearl LH. Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J Med Chem. 1999; 42:260–266. PMID: 9925731.

28. Larsen L, Strling J, Darville M, Eizirik DL, Bonny C, Billestrup N, Mandrup-Poulsen T. Extracellular signal-regulated kinase is essential for interleukin-1-induced and nuclear factor kappaB-mediated gene expression in insulin-producing INS-1E cells. Diabetologia. 2005; 48:2582–2590. PMID: 16283237.
Fig. 1
Inhibition of nitrite production by radicicol in cytokine-mixture (CM)-stimulated pancreatic beta cells. Pancreatic beta cell line, MIN6N8a, was treated with the indicated concentrations of radicicol in the presence of cytokine mixture (CM: TNF-α, 500 U/ml; IFN-γ, 100 U/ml; IL-1β, 10 U/ml) for 48 h. (A) Supernatants were subsequently isolated and analyzed for nitrite. (B) Cells were analyzed for the viablility by 0.4% trypan blue staining. Each column shows the mean±S.D. of triplicate determinations. *Response that is significantly different from the control group as determined by Dunnett's two-tailed t test at p<0.05.
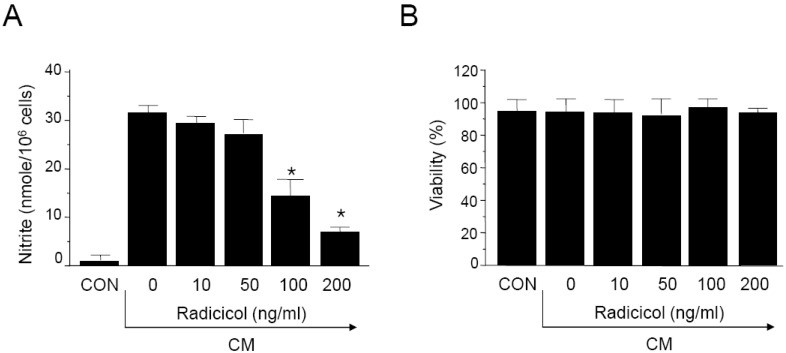
Fig. 2
Inhibition of iNOS gene expression by radicicol in CM-stimulated MIN6N8a cells. (A) MIN6N8a cells were treated with radicicol (100 ng/ml) in the presence of CM for 24 h on a cover slide in 12-well plates. Cells were subjected to immunofluorescence staining using an antibody specific for murine iNOS. Immunoreactivity of iNOS was localized along the margins of the cytoplasm in the control group. MIN6N8a cells were treated with the indicated concentrations of radicicol in the presence of CM for 24 h (B) or 8 h (C). (B) Expression of iNOS was analyzed by Western immunoblotting using antibody specific for murine iNOS. (C) Total RNA was isolated and analyzed for mRNA expression levels of iNOS and β-actin.
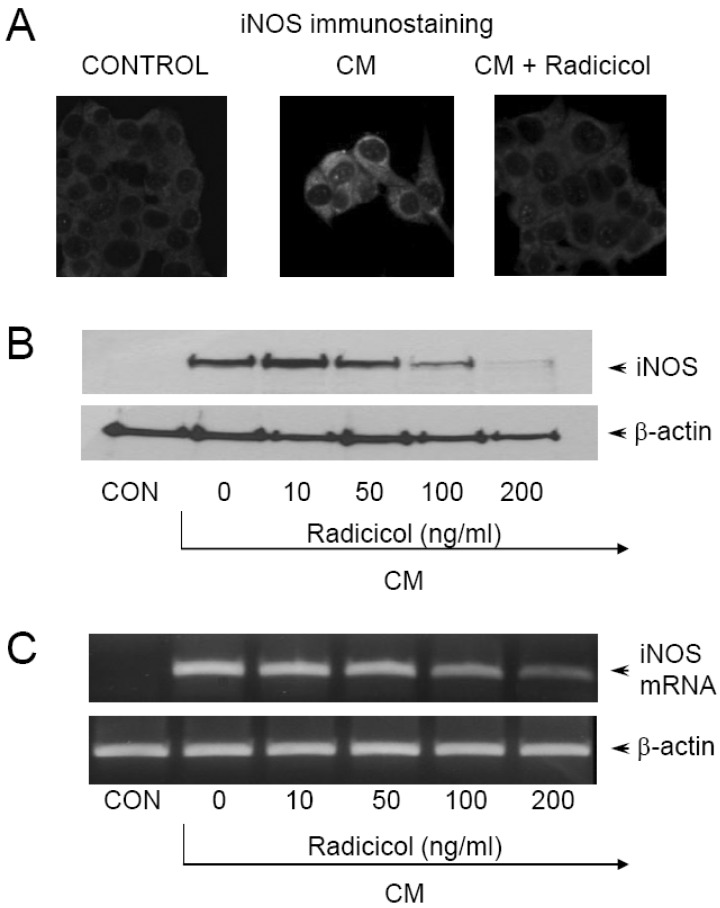
Fig. 3
Inhibition of NF-κB/Rel activation by radicicol in CM-stimulated MIN6N8a cells. (A, B) Inhibition of NF-κB/Rel transcriptional activation by radicicol. MIN6N8a cells were transfected with p(NF-κB/Rel)3-CAT (A) or p(Oct)3-CAT (B) using the DEAE dextran method. Twenty-four hours after transfection, cells were treated with the indicated concentrations of radicicol in the presence of CM for 18 h. Cell extracts were then prepared and analyzed for the expression of CAT using a CAT ELISA kit. (C, D) Inhibition of NF-κB/Rel DNA binding by radicicol. Cells were incubated with radicicol in the presence of CM for 2 h. Nuclear extracts (5 mg/ml) were then isolated and analyzed for the activities of NF-κB/Rel (C) and Oct (D). Each column shows the mean±S.D. of triplicate determinations. *Response that is significantly different from the control group as determined by Dunnett's two-tailed t test at p<0.05.
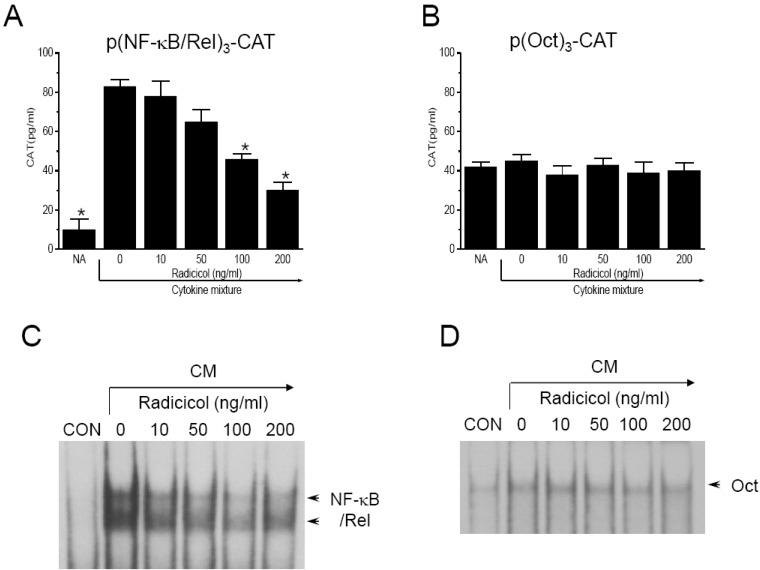
Fig. 4
Inhibition of p65 nuclear translocation by radicicol in CM-stimulated MIN6N8a cells. (A) Cells (5×105 cells/ml) were incubated with radicicol (100 ng/ml) in the presence of CM for 2 h on a cover slide in 12-well plates. Cells were subjected to immunofluorescence staining using an antibody specific for murine p65. One of two representative experiments is shown. (B) Cells were treated with SN50 (10 µM) for 48 h in the presence of CM. The supernatants were subsequently isolated and analyzed for nitrite. Each column shows the mean±S.D. of triplicate determinations. *Response that is significantly different from the control group as determined by Dunnett's two-tailed t test at p<0.05.
Fig. 5
Inhibition of p44/42 phosphorylation by Radicicol in CM-stimulated MIN6N8a cells. (A) MIN6N8a cells were treated with PD98059 (50 µM) or SB203580 (30 µM) for 48 h in the presence of CM. The supernatants were subsequently isolated and analyzed for nitrite. Each column shows the mean±S.D. of triplicate determinations. *Response that is significantly different from the control group as determined by Dunnett's two-tailed t test at p<0.05. (B) Cells were treated with radicicol for the indicated time in the presence of CM. (C) Cells were treated with radicicol for 20 min in the presence of CM. The phosphorylation of p44/p42 was analyzed by Western blot assay.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download