1. Strichartz GR. The inhibition of sodium currents in myelinated nerve by quaternary derivatives of lidocaine. J Gen Physiol. 1973; 62:37–57. PMID:
4541340.

2. Butterworth JF 4th, Strichartz GR. Molecular mechanisms of local anesthesia: a review. Anesthesiology. 1990; 72:711–734. PMID:
2157353.
3. Lee YH, Park NS, Kwon JD, Park JS, Shin GB, Lee CS, Jung TS, Choi NJ, Yoon JH, Ok JS, Yoon UC, Bae MK, Jang HO, Yun I. Amphiphilic effects of dibucaine HCl on rotational mobility of n-(9-anthroyloxy) stearic acid in neuronal and model membranes. Chem Phys Lipids. 2007; 146:33–42. PMID:
17241620.
4. Lee JH, Kim DI, Mun H, Lee SK, Park JS, Kim JH, Lee JH, Park YH, Jeon YC, Yoon UC, Bae MK, Jang HO, Wood WG, Yun I. The effect of propoxycaine.HCl on the physical properties of neuronal membranes. Chem Phys Lipids. 2008; 154:19–25. PMID:
18407836.

5. Seeman P. The membrane actions of anesthetics and tranquilizers. Pharmacol Rev. 1972; 24:583–655. PMID:
4565956.
6. Lee AG. Model for action of local anaesthetics. Nature. 1976; 262:545–548. PMID:
958412.

7. Singer MA. Interaction of dibucaine and propranolol with phospholipid bilayer membranes-effect of alterations in fatty acyl composition. Biochem Pharmacol. 1977; 26:51–57. PMID:
188426.

8. Yun I, Han SK, Baek SW, Kim NH, Kang JS, Chung IK, Lee EJ. Effects of local anesthetics on the fluidity of synaptosomal plasma membrane vesicles isolated from bovine brain. Korean J Pharmacol. 1987; 24:43–52.
9. Smith IC, Auger M, Jarrell HC. Molecular details of anesthetic-lipid interaction. Ann N Y Acad Sci. 1991; 625:668–684. PMID:
2058915.

10. Strichartz GR, Richie JM. The action of local anesthetics on ion channels of excitable tissues. In : Strichartz GR, editor. Local anesthetics. New York: Springer-Verlag;1987. p. 21–52.
11. Lang F, Ritter M, Völkl H, Häussinger D. The biological significance of cell volume. Physiol Ren Biochem. 1993; 16:48–65.

12. Lang F, Busch GL, Ritter M, Völkl H, Waldegger S, Gulbins E, Häussinger D. Functional significance of cell volume regulatory mechanisms. Physiol Rev. 1998; 78:247–306. PMID:
9457175.

13. Roth SH, Miller KW. Roth H, editor. Molecular and Cellular Mechanisms of Anesthetics. New York: Plenum;1986. p. 157–171.
14. Yun I, Kang JS. The general lipid composition and aminophospholipid asymmetry of synaptosomal plasma membrane vesicles isolated from bovine cerebral cortex. Mol Cells. 1990; 1:15–20.
15. Kang JS, Choi CM, Yun I. Effects of ethanol on lateral and rotational mobility of plasma membrane vesicles isolated from cultured mouse myeloma cell line Sp2/0-Ag14. Biochim Biophys Acta. 1996; 1281:157–163. PMID:
8664314.

16. Yun I, Kim YS, Yu SH, Chung IK, Kim IS, Baik SW, Cho GJ, Chung YZ, Kim SH, Kang JS. Comparision of several procedures for the preparation of synaptosomal plasma membrane vesicles. Arch Pharm Res. 1990; 13:325–329.
17. Jang HO, Shin HG, Yun I. Effects of dimyristoylphosphatidylethanol on the structural parameters of neuronal membranes. Mol Cells. 2004; 17:485–491. PMID:
15232224.
18. Jang HO, Jeong DK, Ahn SH, Yoon CD, Jeong SC, Jin SD, Yun I. Effects of chlorpromazine HCl on the structural parameters of bovine brain membranes. J Biochem Mol Biol. 2004; 37:603–611. PMID:
15479625.
19. Bae MK, Jeong DK, Park NS, Lee CH, Cho BH, Jang HO, Yun I. The effect of ethanol on the physical properties of neuronal membranes. Mol Cells. 2005; 19:356–364. PMID:
15995352.
20. Yun I, Cho ES, Jang HO, Kim UK, Choi CH, Chung IK, Kim IS, Wood WG. Amphiphilic effects of local anesthetics on rotational mobility in neuronal and model membranes. Biochim Biophys Acta. 2002; 1564:123–132. PMID:
12101004.

21. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951; 193:265–275. PMID:
14907713.

22. Huang TC, Chen CP, Wefler V, Raftery A. A stable reagent for the Liebermann-Burchard reaction. Anal Chem. 1961; 33:1405–1407.
23. Bartlett GR. Phosphorus assay in column chromatography. J Biol Chem. 1959; 234:466–468. PMID:
13641241.

24. Madeira VMC, Antunes-Madeira MC. Lipid composition of biomembranes: a complete analysis of sarcoplasmic reticulum phospholipids. Cienc Biol (Coimbra). 1976; 2:265–291.
25. Angelova ML, Dimitrov DS. Liposome electroformation. Faraday Discuss Chem Soc. 1986; 81:303–311.

26. Dimitrov DS, Angelova ML. Lipid swelling and liposome formation on solid surfaces in external electric fields. Prog Colloid Polym Sci. 1987; 73:48–56.

27. Angelova ML, Soléau S, Meléard P, Faucon F, Bothorel P. Preparation of giant vesicles by external AC fields, kinetics and application. Prog Colloid Polym Sci. 1992; 89:127–131.
28. Bagatolli LA, Gratton E. Two-photon fluorescence microscopy observation of shape changes at the phase transition in phospholipid giant unilamellar vesicles. Biophys J. 1999; 77:2090–2101. PMID:
10512829.

29. Bagatolli LA, Gratton E. Two photon fluorescence microscopy of coexisting lipid domains in giant unilamellar vesicles of binary phospholipid mixtures. Biophys J. 2000; 78:290–305. PMID:
10620293.

30. Eisinger J, Flores J. Cytosol-membrane interface of human erythrocytes. a resonance energy transfer study. Biophys J. 1983; 41:367–379. PMID:
6838975.
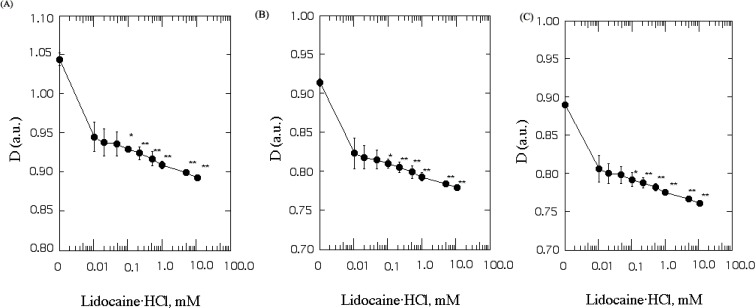
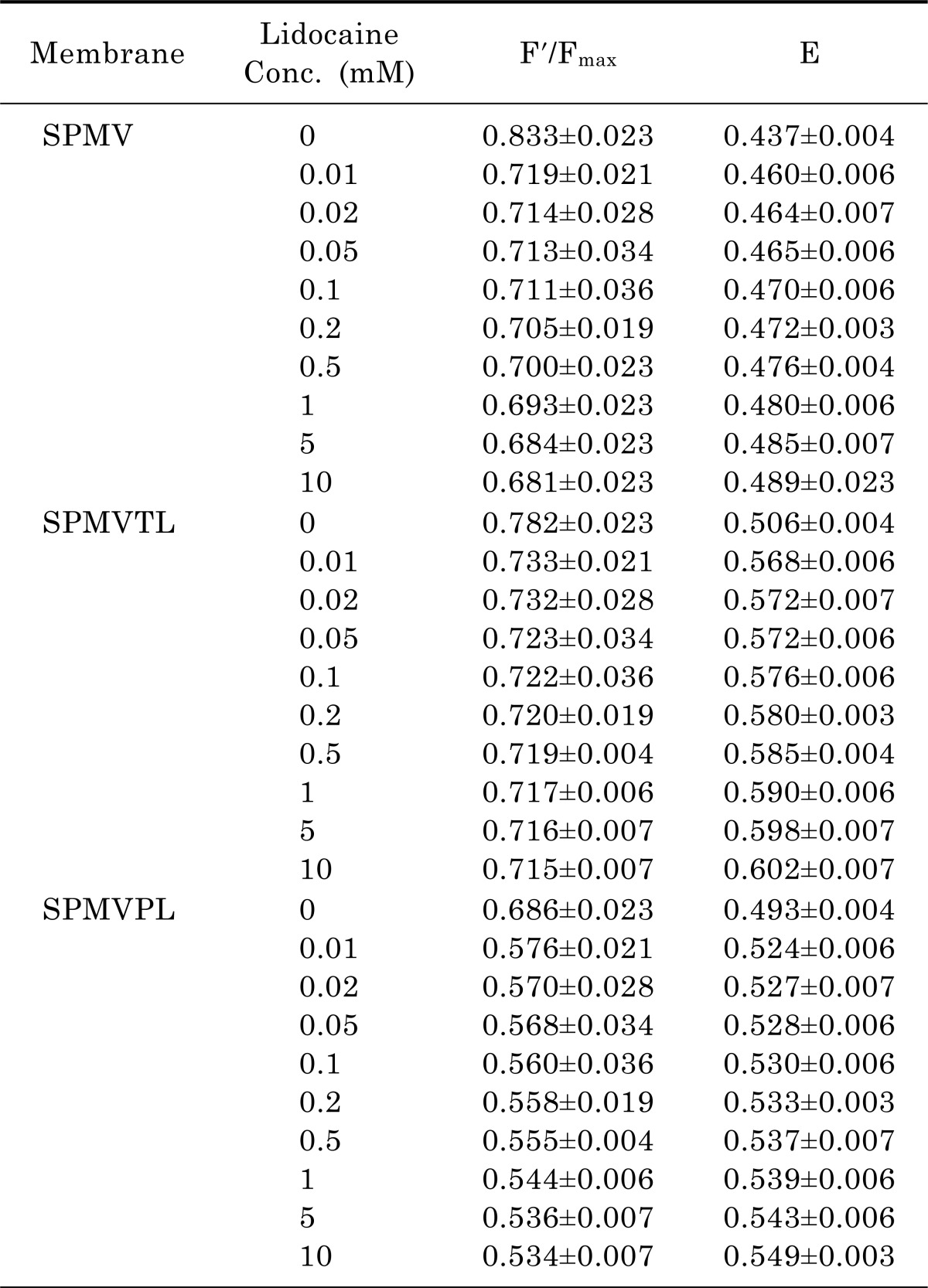
31. Park JS, Jung TS, Noh YH, Kim WS, Park WI, Kim YS, Chung IK, Sohn UD, Bae SK, Bae MK, Jang HO, Yun I. The effect of lidocaine·HCl on the fluidity of native and model membrane lipid bilayers. Korean J Physiol Pharmacol. 2012; 16:413–422. PMID:
23269904.