1. Aller MA, Arias N, Fuentes-Julian S, Blazquez-Martinez A, Argudo S, Miguel MP, Arias JL, Arias J. Coupling inflammation with evo-devo. Med Hypotheses. 2012; 78:721–731. PMID:
22405850.

2. Biasi F, Astegiano M, Maina M, Leonarduzzi G, Poli G. Polyphenol supplementation as a complementary medicinal approach to treating inflammatory bowel disease. Curr Med Chem. 2011; 18:4851–4865. PMID:
21919842.

3. Kim JK, Jun JG. Ailanthoidol suppresses lipopolysaccharidestimulated inflammatory reactions in RAW264.7 cells and endotoxin shock in mice. J Cell Biochem. 2011; 112:3816–3823. PMID:
21826708.

4. Lee YJ, Kao ES, Chu CY, Lin WL, Chiou YH, Tseng TH. Inhibitory effect of ailanthoidol on 12-O-tetradecanoyl-phorbol-13-acetate-induced tumor promotion in mouse skin. Oncol Rep. 2006; 16:921–927. PMID:
16969515.

5. Lee WS, Baek YI, Kim JR, Cho KH, Sok DE, Jeong TS. Antioxidant activities of a new lignan and a neolignan from Saururus chinensis. Bioorg Med Chem Lett. 2004; 14:5623–5628. PMID:
15482936.

6. Abdel-Wahab BF, Abdel-Aziz HA, Ahmed EM. Synthesis and antimicrobial evaluation of 1-(benzofuran-2-yl)-4-nitro-3-arylbutan-1-ones and 3-(benzofuran-2-yl)-4,5-dihydro-5-aryl-1-[4-(aryl)-1,3-thiazol-2-yl]-1H-pyrazoles. Eur J Med Chem. 2009; 44:2632–2635. PMID:
18995932.

7. Lin SY, Chen CL, Lee YJ. Total synthesis of ailanthoidol and precursor XH14 by Stille coupling. J Org Chem. 2003; 68:2968–2971. PMID:
12662079.

8. Rao MLN, Awasthi DK, Banerjee D. An expeditious and convergent synthesis of ailanthoidol. Tetrahedron Lett. 2010; 51:1979–1981.

9. Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010; 10:826–837. PMID:
21088683.

10. Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009; 27:451–483. PMID:
19105661.

11. Natoli G, Ghisletti S, Barozzi I. The genomic landscapes of inflammation. Genes Dev. 2011; 25:101–106. PMID:
21245163.

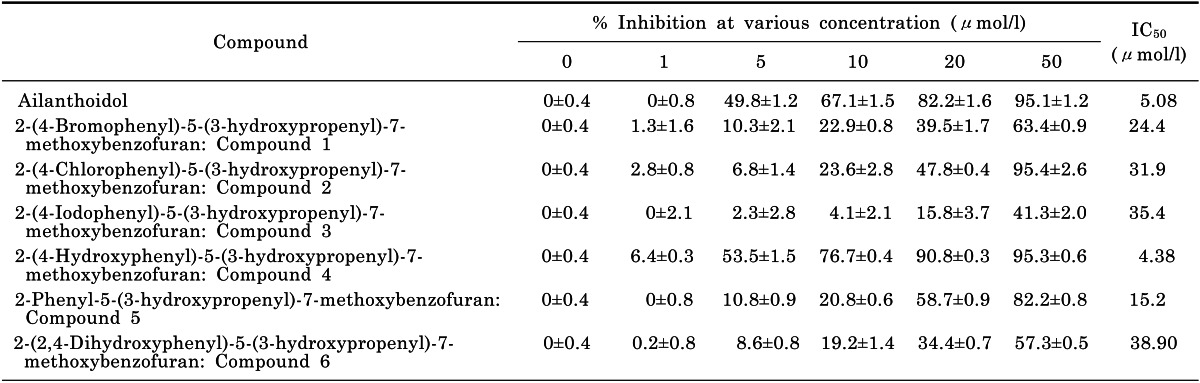
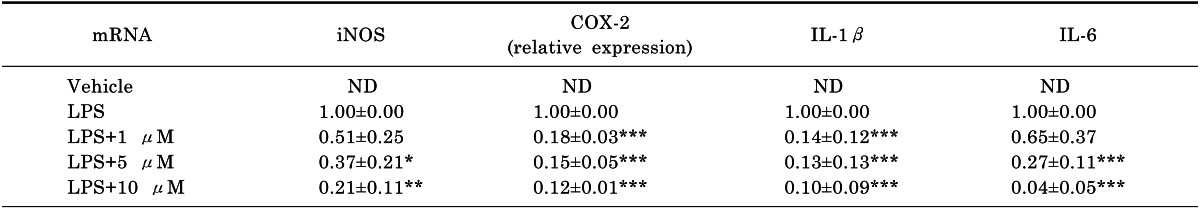
12. Lee LR, Lee JJ, Kim , Jun JG. Ailanthoidol derivatives and their anti-inflammatory effects. Bull Korean Chem Soc. 2012; 33:1907–1912.

13. Chen CY, Peng WH, Tsai KD, Hsu SL. Luteolin suppresses inflammation-associated gene expression by blocking NF-kappaB and AP-1 activation pathway in mouse alveolar macrophages. Life Sci. 2007; 81:1602–1614. PMID:
17977562.
14. Shaulian E. AP-1--The Jun proteins: Oncogenes or tumor suppressors in disguise? Cell Signal. 2010; 22:894–899. PMID:
20060892.
15. Kawada N, Moriyama T, Kitamura H, Yamamoto R, Furumatsu Y, Matsui I, Takabatake Y, Nagasawa Y, Imai E, Wilcox CS, Rakugi H, Isaka Y. Towards developing new strategies to reduce the adverse side-effects of nonsteroidal anti-inflammatory drugs. Clin Exp Nephrol. 2012; 16:25–29. PMID:
22038259.

16. Li C, Lin G, Zuo Z. Pharmacological effects and pharmacokinetics properties of Radix Scutellariae and its bioactive flavones. Biopharm Drug Dispos. 2011; 32:427–445. PMID:
21928297.

17. Pontiki E, Hadjipavlou-Litina D, Litinas K, Nicolotti O, Carotti A. Design, synthesis and pharmacobiological evaluation of novel acrylicacid derivatives acting as lipoxygenase and cyclooxygenase-1 inhibitors with antioxidant and anti-inflammatory activities. Eur J Med Chem. 2011; 46:191–200. PMID:
21106277.
18. Altavilla D, Squadrito F, Bitto A, Polito F, Burnett BP, Di Stefano V, Minutoli L. Flavocoxid, a dual inhibitor of cyclooxygenase and 5-lipoxygenase, blunts pro-inflammatory phenotype activation in endotoxin-stimulated macrophages. Br J Pharmacol. 2009; 157:1410–1418. PMID:
19681869.

19. Sharma N, Mohanakrishnan D, Shard A, Sharma A, Saima , Sinha AK, Sahal D. Stilbene-chalcone hybrids: design, synthesis, and evaluation as a new class of antimalarial scaffolds that trigger cell death through stage specific apoptosis. J Med Chem. 2012; 55:297–311. PMID:
22098429.

20. Karapetyan G, Chakrabarty K, Hein M, Langer P. Synthesis and bioactivity of carbohydrate derivatives of indigo, its isomers and heteroanalogues. Chem Med Chem. 2011; 6:25–37. PMID:
21108279.

21. Silvia V, Baldisserotto A, Scalambra E, Malisardi G, Durini E, Manfredini S. Novel molecular combination deriving from natural aminoacids and polyphenols: Design, synthesis and free-radical scavenging activities. Eur J Med Chem. 2012; 50:383–392. PMID:
22385675.

22. Fokialakis N, Magiatis P, Mitaku S, Pratsinis H, Tillequin F. Estrogenic activity of phenylpropanoids from Sarcomelicope megistophylla and structure determination of a new norneolignan. Planta Med. 2003; 69:566–568. PMID:
12865982.
23. Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011; 11:723–737. PMID:
21997792.

24. Vesely PW, Staber PB, Hoefler G, Kenner L. Translational regulation mechanisms of AP-1 proteins. Mutat Res. 2009; 682:7–12. PMID:
19167516.

25. Pasparakis M. Regulation of tissue homeostasis by NF-kappaB signalling: implications for inflammatory diseases. Nat Rev Immunol. 2009; 9:778–788. PMID:
19855404.
26. Schonthaler HB, Guinea-Viniegra J, Wagner EF. Targeting inflammation by modulating the Jun/AP-1 pathway. Ann Rheum Dis. 2011; 70:i109–i112. PMID:
21339212.