1. Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999. 38:1083–1152.
2. Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004. 44:5–21.
3. Dori I, Dinopoulos A, Blue ME, Parnavelas JG. Regional differences in the ontogeny of the serotonergic projection to the cerebral cortex. Exp Neurol. 1996. 138:1–14.
4. Kojic L, Dyck RH, Gu Q, Douglas RM, Matsubara J, Cynader MS. Columnar distribution of serotonin-dependent plasticity within kitten striate cortex. Proc Natl Acad Sci USA. 2000. 97:1841–1844.
5. Kirkwood A. Serotonergic control of developmental plasticity. Proc Natl Acad Sci USA. 2000. 97:1951–1952.
6. Rittenhouse CD, Shouval HZ, Paradiso MA, Bear MF. Monocular deprivation induces homosynaptic long-term depression in visual cortex. Nature. 1999. 397:347–350.
7. Bear MF, Rittenhouse CD. Molecular basis for induction of ocular dominance plasticity. J Neurobiol. 1999. 41:83–91.
8. Fagiolini M, Katagiri H, Miyamoto H, Mori H, Grant SG, Mishina M, Hensch TK. Separable features of visual cortical plasticity revealed by N-methyl-D-aspartate receptor 2A signaling. Proc Natl Acad Sci USA. 2003. 100:2854–2859.
9. Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005. 6:877–888.
10. Gu Q, Singer W. Involvement of serotonin in developmental plasticity of kitten visual cortex. Eur J Neurosci. 1995. 7:1146–1153.
11. Kim HS, Jang HJ, Cho KH, Hahn SJ, Kim MJ, Yoon SH, Jo YH, Kim MS, Rhie DJ. Serotonin inhibits the induction of NMDA receptor-dependent long-term potentiation in the rat primary visual cortex. Brain Res. 2006. 1103:49–55.
12. Jang HJ, Cho KH, Park SW, Kim MJ, Yoon SH, Rhie DJ. Effects of serotonin on the induction of long-term depression in the rat visual cortex. Korean J Physiol Pharmacol. 2010. 14:337–343.
13. Edagawa Y, Saito H, Abe K. Endogenous serotonin contributes to a developmental decrease in long-term potentiation in the rat visual cortex. J Neurosci. 2001. 21:1532–1537.
14. Edagawa Y, Saito H, Abe K. The serotonin 5-HT2 receptor-phospholipase C system inhibits the induction of long-term potentiation in the rat visual cortex. Eur J Neurosci. 2000. 12:1391–1396.
15. Edagawa Y, Saito H, Abe K. 5-HT1A receptor-mediated inhibition of long-term potentiation in rat visual cortex. Eur J Pharmacol. 1998. 349:221–224.
16. Staubli U, Otaky N. Serotonin controls the magnitude of LTP induced by theta bursts via an action on NMDA-receptor-mediated responses. Brain Res. 1994. 643:10–16.
17. Pollandt S, Drephal C, Albrecht D. 8-OH-DPAT suppresses the induction of LTP in brain slices of the rat lateral amygdala. Neuroreport. 2003. 14:895–897.
18. Ohashi S, Matsumoto M, Togashi H, Ueno K, Yoshioka M. The serotonergic modulation of synaptic plasticity in the rat hippocampo-medial prefrontal cortex pathway. Neurosci Lett. 2003. 342:179–182.
19. Maya Vetencourt JF, Sale A, Viegi A, Baroncelli L, De Pasquale R, O'Leary OF, Castrén E, Maffei L. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science. 2008. 320:385–388.
20. Maya Vetencourt JF, Tiraboschi E, Spolidoro M, Castrén E, Maffei L. Serotonin triggers a transient epigenetic mechanism that reinstates adult visual cortex plasticity in rats. Eur J Neurosci. 2011. 33:49–57.
21. Li QH, Nakadate K, Tanaka-Nakadate S, Nakatsuka D, Cui Y, Watanabe Y. Unique expression patterns of 5-HT2A and 5-HT2C receptors in the rat brain during postnatal development: Western blot and immunohistochemical analyses. J Comp Neurol. 2004. 469:128–140.
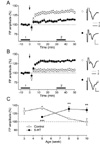
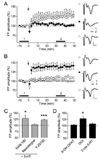
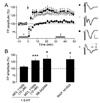
22. Jang HJ, Cho KH, Kim HS, Hahn SJ, Kim MS, Rhie DJ. Age-dependent decline in supragranular long-term synaptic plasticity by increased inhibition during the critical period in the rat primary visual cortex. J Neurophysiol. 2009. 101:269–275.
23. Kirkwood A, Silva A, Bear MF. Age-dependent decrease of synaptic plasticity in the neocortex of alphaCaMKII mutant mice. Proc Natl Acad Sci USA. 1997. 94:3380–3383.
24. Jang HJ, Cho KH, Park SW, Kim MJ, Yoon SH, Rhie DJ. The development of phasic and tonic inhibition in the rat visual cortex. Korean J Physiol Pharmacol. 2010. 14:399–405.
25. Moreau AW, Amar M, Le Roux N, Morel N, Fossier P. Serotoninergic fine-tuning of the excitation-inhibition balance in rat visual cortical networks. Cereb Cortex. 2010. 20:456–467.
26. Hannon J, Hoyer D. Molecular biology of 5-HT receptors. Behav Brain Res. 2008. 195:198–213.
27. Morales M, Bloom FE. The 5-HT3 receptor is present in different subpopulations of GABAergic neurons in the rat telencephalon. J Neurosci. 1997. 17:3157–3167.
28. Feng J, Cai X, Zhao J, Yan Z. Serotonin receptors modulate GABAA receptor channels through activation of anchored protein kinase C in prefrontal cortical neurons. J Neurosci. 2001. 21:6502–6511.
29. Kirkwood A, Lee HK, Bear MF. Co-regulation of long-term potentiation and experience-dependent synaptic plasticity in visual cortex by age and experience. Nature. 1995. 375:328–331.
30. Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999. 98:739–755.
31. Hensch TK, Fagiolini M, Mataga N, Stryker MP, Baekkeskov S, Kash SF. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science. 1998. 282:1504–1508.
32. Kirkwood A, Bear MF. Hebbian synapses in visual cortex. J Neurosci. 1994. 14:1634–1645.
33. Kirkwood A, Rozas C, Kirkwood J, Perez F, Bear MF. Modulation of long-term synaptic depression in visual cortex by acetylcholine and norepinephrine. J Neurosci. 1999. 19:1599–1609.
34. Bear MF, Singer W. Modulation of visual cortical plasticity by acetylcholine and noradrenaline. Nature. 1986. 320:172–176.
35. Kojic L, Gu Q, Douglas RM, Cynader MS. Serotonin facilitates synaptic plasticity in kitten visual cortex: an in vitro study. Brain Res Dev Brain Res. 1997. 101:299–304.
36. Bröcher S, Artola A, Singer W. Agonists of cholinergic and noradrenergic receptors facilitate synergistically the induction of long-term potentiation in slices of rat visual cortex. Brain Res. 1992. 573:27–36.
37. Markram H, Segal M. Long-lasting facilitation of excitatory postsynaptic potentials in the rat hippocampus by acetylcholine. J Physiol. 1990. 427:381–393.
38. Wang Y, Gu Q, Cynader MS. Blockade of serotonin-2C receptors by mesulergine reduces ocular dominance plasticity in kitten visual cortex. Exp Brain Res. 1997. 114:321–328.
39. Gordon B, Mitchell B, Mohtadi K, Roth E, Tseng Y, Turk F. Lesions of nonvisual inputs affect plasticity, norepinephrine content, and acetylcholine content of visual cortex. J Neurophysiol. 1990. 64:1851–1860.
40. Jang HJ, Cho KH, Park SW, Kim MJ, Yoon SH, Rhie DJ. Layer-specific serotonergic facilitation of IPSC in layer 2/3 pyramidal neurons of the visual cortex. J Neurophysiol. 2012. 107:407–416.
41. Mattson MP, Maudsley S, Martin B. BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2004. 27:589–594.
42. Nakamura T, Nakamura K, Lasser-Ross N, Barbara JG, Sandler VM, Ross WN. Inositol 1,4,5-trisphosphate (IP3)-mediated Ca2+ release evoked by metabotropic agonists and backpropagating action potentials in hippocampal CA1 pyramidal neurons. J Neurosci. 2000. 20:8365–8376.
43. Jiang B, Huang ZJ, Morales B, Kirkwood A. Maturation of GABAergic transmission and the timing of plasticity in visual cortex. Brain Res Brain Res Rev. 2005. 50:126–133.