Abstract
Glutamate excitotoxicity is emerging as a contributor to degeneration of spinal cord motoneurons in amyotrophic lateral sclerosis (ALS). Recently, we have reported that ghrelin protects motoneurons against chronic glutamate excitotoxicity through the activation of extracellular signal-regulated kinase 1/2 and phosphatidylinositol-3-kinase/Akt/glycogen synthase kinase-3β pathways. Previous studies suggest that activated microglia actively participate in the pathogenesis of ALS motoneuron degeneration. However, it is still unknown whether ghrelin exerts its protective effect on motoneurons via inhibition of microglial activation. In this study, we investigate organotypic spinal cord cultures (OSCCs) exposed to threohydroxyaspartate (THA), as a model of excitotoxic motoneuron degeneration, to determine if ghrelin prevents microglial activation. Exposure of OSCCs to THA for 3 weeks produced typical motoneuron death, and treatment of ghrelin significantly attenuated THA-induced motoneuron loss, as previously reported. Ghrelin prevented THA-induced microglial activation in the spinal cord and the expression of pro-inflammatory cytokines tumor necrosis factor-α and interleukin-1β. Our data indicate that ghrelin may act as a survival factor for motoneurons by functioning as a microglia-deactivating factor and suggest that ghrelin may have therapeutic potential for the treatment of ALS and other neurodegenerative disorders where inflammatory responses play a critical role.
Amyotrophic lateral sclerosis (ALS) is a chronic, progressive neurodegenerative disease for which there is no cure. Individuals with ALS are characterized by the selective loss of motoneurons in the spinal cord, brain stem and motor cortex, which results in a progressive muscle weakness and paralysis [1]. Despite extensive research, the mechanism causing motoneuron death in ALS is still mostly unclear; however, glutamate excitotoxicity has been implicated in neurodegeneration in this disease [2]. Glutamate re-uptake is diminished in synaptosomes from cerebral cortex and spinal cord of ALS patients [3] due to a selective loss of the astroglial glutamate transporter in the motor cortex and spinal cord [4]. These changes result in an excessive concentration of glutamate in the synaptic cleft and the excessive stimulation of glutamate receptors give rise to an increased intracellular concentration of Na+ and Ca2+, and then this can cause excitotoxic neuronal death [2]. Another common characteristic of ALS is the occurrence of neuroinflammatory reaction [5]. The accumulation of reactive microglia is detected in degenerating areas of ALS patients and transgenic animal models [6,7]. A variety of noxious compounds, such as reactive oxygen species (ROS), reactive nitrogen species, pro-inflammatory cytokines, and prostaglandins, released from inappropriately activated microglia, stimulate ionotropic glutamate receptors leading to enhanced neuronal damage [8-10]. Considering that minocycline, a semisynthetic tetracycline derivatives, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia [11,12], the molecules targeting pharmacological intervention of microglial activation may be plausible therapeutic candidates for neuroprotective agents in ALS.
Ghrelin, a unique peptide esterified with octanoic acid on Ser 3 [13], is a novel 28-amino acid hormone that is principally synthesized and released from Gr cells in the oxyntic mucosa of the stomach [14]. In addition to its potent stimulating effect on growth hormone (GH) release from the anterior pituitary gland, ghrelin induces a positive energy balance by increasing food intake while decreasing fat use through GH-independent mechanisms [15,16]. Ghrelin also exerts numerous peripheral effects including direct effects on exocrine and endocrine pancreatic functions, carbohydrate metabolism, the cardiovascular system, gastric secretion, stomach motility, and sleep [13]. We have demonstrated that ghrelin exerts neuroprotective effects in different in vivo and in vitro disease models in which excitotoxicity has been involved, including stroke [17-19] and Parkinson's disease [20]. Moreover, we recently have reported that ghrelin protects spinal cord motoneurons after glutamate excitotoxicity through the activation of extracellular signal-regulated kinase (ERK) 1/2 and phosphatidylinositol-3-kinase (PI3K)/Akt/glycogen synthase kinase (GSK)-3β pathways [21]. However, it is still unknown whether ghrelin exerts its protective effect in chronic glutamate-induced excitotoxic death of spinal cord motoneurons via inhibition of microglial activation. Given the accumulation of reactive microglia in degenerative areas is one of the cellular events signaling the presence of neuroinflammation in ALS [22] and ghrelin inhibits 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)- and kainic acid (KA)-induced microglial activation [20,23], these findings suggest that ghrelin may protect spinal cord motoneurons after glutamate excitotoxicity through the inhibition of microglial activation. Therefore, in the current study, we investigated if ghrelin treatment prevents microglial activation in chronic glutamate-induced excitotoxicity model using organotypic spinal cord cultures (OSCCs) exposed to an inhibitor of glutamate transport, threohydroxyaspartate (THA).
Rat ghrelin was obtained from Peptides International (Louisville, KT, USA). Monoclonal antibodies to mouse anti-non-phosphorylated neurofilament H (SMI-32) was purchased from Covance (Princeton, NJ, USA), rat anti-Mac-1 from Chemicon (Carlsbad, CA, USA), and goat anti-tumor necrosis factor (TNF)-α and anti-interleukin (IL)-1β from R&D Systems (Minneapolis, MN, USA). All tissue culture reagents were obtained from Gibco/Invitrogen, and all other reagents were obtained from Sigma (St. Louis, MO, USA) unless otherwise indicated.
OSCCs were prepared from lumbar spinal cords of 8-day-old Sprague-Dawley rat pups (P8), as previously described [24]. Briefly, pups were decapitated and the lumbar spinal cords were collected under sterile conditions and transferred to sterile Gey's balanced salt solution containing glucose (6.4 mg/ml). They were transversely sectioned at 350 µm by using a McIIwain Tissue Chopper (Mickle Laboratory Engineering, Gomshall, Surrey, UK). Five or six complete slices were transferred to 30-mm-diameter Millipore Millicell®-CM culture plate inserts (0.4 µm, Millipore, Carrightwohill, Co. Cork, Ireland). The inserts were placed in six-well culture plates (35-mm-diameter, Falcon, Becton-Dickinson, Franklin Lakes, NJ, USA), with 1 ml culture medium: 50% (vol/vol) minimal essential medium with 25 mM Hepes, 25% (vol/vol) heat-inactivated horse serum and 25% (vol/vol) Hank's balanced salt solution supplemented with D-glucose (25.6 mg/ml, Sigma), 2 mM L-glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin (Gibco/Invitrogen). Cultures were incubated at 37℃ in a 5% CO2/95% air humidified incubator. Medium was changed twice every week. Under these conditions, cultures can be maintained up to 3 months with steady motoneuron survival and preservation of synaptic morphology. All experiments started at least 7 days after the explant procedure. Chronic glutamate excitotoxicity was induced by exposure to THA (for 1 to 3 weeks), a potent glutamate transporter inhibitor, which is known to produce a dose-dependent sustained elevation of glutamate levels causing degeneration of motoneurons [25]. To determine whether ghrelin inhibits THA-induced motoneuron death or microglial activation in OSCCs, cultures were treated with ghrelin (100 nM) or vehicle at the same time as THA. In this study, 100 nM of ghrelin was chosen on the basis of our previous study that this dose of ghrelin exerted a potent protective effect on spinal cord motoneurons [21]. All experiments were performed three times, giving essentially identical results.
Motoneurons in the OSCCs were identified by SMI-32 immunostaining and on the basis of their morphology and size (>25 µm) and their localization in the ventral horn. All motoneurons meeting these criteria were blindly counted in each spinal cord section. A minimum of 15 sections was counted for each experimental condition.
OSCCs growing in the different experimental conditions were fixed with 4% paraformaldehyde (Sigma) in phosphate-buffered saline for 30 min at room temperature. After blocking with 3% normal goat serum (Vector Laboratories, Burlingame, CA, USA) and 1% bovine serum albumin (BSA) (Sigma), the sections were incubated with primary antibodies to SMI-32 (1:8,000), Mac-1 (1:500), TNF-α (1:200), or IL-1β (1:100) overnight at 4℃. For immunofluorescence, sections were incubated for 1.5 h at room temperature with the appropriate secondary antibody, biotinylated anti-goat IgG (1:2,000; Vector Laboratories) or cy3-conjugated goat anti-rat IgG (1:500, Jackson Immuno-Research, West Grove, PA, USA). Sections were counterstained with DAPI and fluorescence was observed using a Zeiss LSM 510 Meta confocal microscope (Carl Zeiss, Oberkochen, Germany).
The production of TNF-α and IL-1β in the OSCCs was determined in culture supernatants using specific ELISA kits (BioSource International, Inc., Camarillo, CA, USA) according to the manufacturer's instructions.
Data are presented as mean±SEM. Statistical analysis between groups was performed using 1-way ANOVA and Holm-Sidak method for multiple comparisons using SigmaStat for Windows Version 3.10 (Systat Software, Inc. Point Richmond, CA, USA). p<0.05 was considered statistically significant.
To investigate the effects of ghrelin on spinal cord motoneurons exposed to chronic glutamate-induced excitotoxicity, OSCCs were incubated with THA, a glutamate-uptake inhibitor, in the presence or absence of ghrelin. After 3-week exposure of OSCC to THA (100 µM), 58.8% of motoneurons were not viable as determined by SMI-32 immunohistochemistry. However, THA-induced motoneuron death was significantly reduced by treatment of ghrelin (100 nM) (Fig. 1), as we previously reported [21].
It has been reported that a neuroinflammatory response precedes motoneuron death caused by THA-induced glutamate excitotoxicity [26]. To investigate whether the protective effect of ghrelin on spinal cord motoneurons is associated with the inhibition of the THA-induced microglial activation, we examined the expression of Mac-1, which is a specific marker for microglial activation [10]. Immunohistochemical analysis showed that THA resulted in a significant increase in the immunoreactivity of activated microglia after 1 week of treatment with THA and lasted for 3 weeks (Fig. 2). However, in THA- and ghrelin-treated cultures, ventral horn Mac-1 immunoreactivities were significantly reduced when compared with THA- and vehicle-treated group (Fig. 2).
It is well known that neuroinflammation plays important roles in motoneuron damage in ALS [5,9]. Pro-inflammatory mediators released from activated microglia could be a link between neuroinflammation and excitotoxic spinal cord motoneuron death [5,9]. Given the fact that ghrelin suppressed the THA-induced microglial response, we investigated whether the release and expression of pro-inflammatory cytokines from activated microglial cells is inhibited by ghrelin treatment. Fig. 3A shows that the levels of TNF-α released into the culture medium were increased after 1-week exposure to THA and lasted for 3 weeks. Additionally, after 2-week exposure of cultures to THA, double immunostaining of OSCCs with Mac-1 and TNF-α antibodies showed that THA resulted in a significant increase in TNF-α immunoreactivities and most of TNF-α-positive cells colocalized to microglial cells (Fig. 3B). However, similar to the suppression of microglial activation, ghrelin treatment significantly inhibited the THA-induced increase of TNF-α (Fig. 3). We also performed ELISA analysis and immunohistochemistry to investigate the effect of ghrelin on THA-induced IL-1β release and expression in the OSCCs and obtained similar results (Fig. 4).
In the present study, we show that ghrelin treatment reduces spinal cord motoneuron death caused by chronic glutamate-induced excitotoxicity, as previously described [21]. The protective effect of ghrelin on motoneurons appears to be mediated by the inhibition of microglial activation and concomitant release of microglia-derived pro-inflammatory mediators.
As we recently reported [21], ghrelin protects spinal cord motoneurons from THA-induced chronic glutamate excitotoxicity in OSCCs. It has been shown that ghrelin exerts its effect through the activation of its receptor GH secretagogue receptor (GHS-R) 1a and activates ERK1/2 and Akt in motoneurons. Ghrelin-induced Akt signaling was also associated with downstream inhibition of GSK-3β in motoneurons [21]. Although these findings suggest a direct role of ghrelin on spinal cord motoneurons, there is a possibility that the neuroprotective effect of ghrelin may be mediated through the influence on non-neuronal cells, such as microglia. As mediators of inflammation, reactive microgliosis is a considerable hallmark of ALS [6,7] and the intensity of microglial activation is correlated with severity of motoneuron damage in human ALS [9], suggesting an active involvement of microglial activation in the disease. Furthermore, we recently demonstrated that ghrelin prevented microglial activation induced by MPTP and KA [20,23]. In agreement with these findings, we show here that ghrelin significantly reduces THA-induced accumulation of reactive microglia in the spinal cord. In that minocycline inhibits the activation and proliferation of microglial cells and protects motoneurons against excitotoxicity [11,12], the inhibitory effect of ghrelin on microglial activation may play an important role in the neuroprotective effect of ghrelin in chronic glutamate excitotoxicity. Additionally, ghrelin may have a direct effect on spinal cord oligodendrocytes because GHS-R1a is expressed in these cells and ghrelin inhibits apoptotic cell death of oligodendrocytes after spinal cord injury [27]. However, the precise role of ghrelin on oligodendrocytes remains to be determined.
Microglia are the primary immune cells of the central nervous system [28]. Activated microglia are capable of producing a variety of noxious compounds, such as ROS (superoxide, nitric oxide, and its derivatives), cytokines, and eicosanoids [8-10]. Pro-inflammatory cytokine levels, including TNF-α and IL-1β, are increased in the central nervous system of ALS patients and in mutant superoxide dismutase 1 transgenic mice [5,9]. These cytokines may increase the susceptibility of motoneurons to glutamate toxicity and inhibit the function and expression of astrocytic glutamate transporters resulting in further neurotoxicity [8]. Consistent with the concept that ghrelin inhibits the THA-induced microglial activation, we found that ghrelin attenuated the expression of TNF-α and IL-1β in the spinal cord. This inhibitory effect of ghrelin on TNF-α and IL-1β may contribute to alleviating THA-induced inflammatory damage. Supporting evidence for this observation is that ghrelin shows anti-inflammatory effect in the periphery [29-31] and in a central hemorrhage model of brain damage [32]. Given the fact that the neuroprotective effect of ghrelin in spinal cord is associated with the inhibition of THA-induced microglial activation, and the subsequent increase in TNF-α and IL-1β, we suggest that ghrelin functions as a microglia-deactivating factor.
Neuroprotective effects of ghrelin have been observed in numerous in vitro and in vivo models of neurodegenerative disorders, including Parkinson's disease [20,33,34], stroke [17-19], epilepsy [23,35], and ALS [21]. It has been reported that both acylated and des-acyl ghrelin produce neuroprotective effects [18,19]. Both types of ghrelin activate similar intracellular signaling pathways, such as ERK1/2, PI3K/Akt, protein kinase A, and proteinase kinase C pathways [18]. It is interesting that des-acyl ghrelin has neuroprotective effects independent of ghrelin receptor GHS-R1a [18,19]. Activation of these signaling pathways results in the inhibition of apoptotic cascades through the increase in the Bcl-2/Bax ratio, the prevention of cytochrome c release and the inhibition of caspase 3 activation [17-19,27]. Ghrelin prevents ischemia induced activation of p-38 and c-Jun NH2-terminal kinase, which activate the pro-apoptotic events [17]. Moreover, ghrelin suppresses neuroinflammation via the inhibition of microglial activation and subsequent release of pro-inflammatory cytokines [20,23]. In addition, ghrelin promotes and protects nigrostriatal dopamine function via a uncoupling protein 2-dependent mitochondrial mechanisms [33]. Taken together, these findings provide evidence that ghrelin acts as a survival factor for neurons, including spinal cord motoneurons, and offer a new perspective on the potential role of this peptide in various neurodegenerative diseases.
In summary, we found that excitotoxic death of motoneurons caused by THA was associated with a neuroinflammatory response characterized by the presence of activated microglia and the increased release of pro-inflammatory mediators, TNF-α and IL-1β, as previously described [26]. We provide the evidence that the neuroprotective effect of ghrelin is mediated by the inhibition of microglial activation and the concomitant release of pro-inflammatory cytokines in the spinal cord. These findings are significant because ghrelin can function as a neuroprotective agent and may have therapeutic potential for the treatment of ALS and other neurodegenerative disorders where inflammatory responses play a critical role.
Figures and Tables
Fig. 1
Ghrelin protects spinal cord motoneurons against THA-induced chronic glutamate excitotoxicity. Spinal cord cultures were treated for 3 weeks with 100 µM THA. Either vehicle or ghrelin (100 nM) was added to the culture medium at the same time as THA. Motoneurons were identified by SMI-32 immunostaining and on the basis of their morphology, size and location. Representative micrographs of SMI-32-(+) motoneurons in the ventral horn of the spinal cord are shown. Scale bar represents 100 µm. Values are the mean±SEM of at least 15 sections per treatment. Each experiment was repeated three times. *p<0.05 vs. control cultures; †p<0.05 vs. THA-insulted, vehicle-treated cultures.
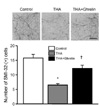
Fig. 2
Ghrelin suppresses THA-induced microglial activation in organotypic spinal cord cultures. Spinal cord cultures were treated for 1 to 3 weeks with 100 µM THA. Either vehicle or ghrelin (100 nM) was added to the culture medium at the same time as THA. (A) The intensity of Mac-1 immunostaining was measured and presented as percent of control (mean±SEM). (B) Representative photomicrographs of Mac-1 immunostaining in each group are shown. Scale bar represents 100 µm. Each experiment was repeated three times. *p<0.05 vs. control cultures; †p<0.05 vs. THA-insulted, vehicle-treated cultures.
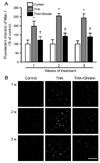
Fig. 3
Ghrelin inhibits the production of TNF-α from activated microglia in organotypic spinal cord cultures. Spinal cord cultures were treated for 1 to 3 weeks with 100 µM THA. Either vehicle or ghrelin (100 nM) was added to the culture medium at the same time as THA. (A) Levels of TNF-α were determined in the media by ELISA. Values are the mean±SEM. (B) Representative photomicrographs of double immunofluorescence with anti-Mac-1 and anti-TNF-α after 2-week exposure to THA. Scale bar represents 100 µm. Each experiment was repeated three times. *p<0.05 vs. control cultures; †p<0.05 vs. THA-insulted, vehicle-treated cultures.
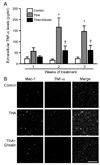
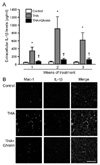
Fig. 4
Ghrelin inhibits the production of IL-1β from activated microglia in organotypic spinal cord cultures. Spinal cord cultures were treated for 1 to 3 weeks with 100 µM THA. Either vehicle or ghrelin (100 nM) was added to the culture medium at the same time as THA. (A) Levels of IL-1β were determined in the media by ELISA. Values are the mean±SEM. (B) Representative photomicrographs of double immunofluorescence with anti-Mac-1 and anti-IL-1β after 2-week exposure to THA. Scale bar represents 100 µm. Each experiment was repeated three times. *p<0.05 vs. control cultures; †p<0.05 vs. THA-insulted, vehicle-treated cultures.
ACKNOWLEDGEMENTS
This study was supported by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MEST) (No. 2011-0002700).
References
1. Goodall EF, Morrison KE. Amyotrophic lateral sclerosis (motor neuron disease): proposed mechanisms and pathways to treatment. Expert Rev Mol Med. 2006. 8:1–22.
2. Van Den Bosch L, Van Damme P, Bogaert E, Robberecht W. The role of excitotoxicity in the pathogenesis of amyotrophic lateral sclerosis. Biochim Biophys Acta. 2006. 1762:1068–1082.
3. Rothstein JD, Martin LJ, Kuncl RW. Decreased glutamate transport by the brain and spinal cord in amyotrophic lateral sclerosis. N Engl J Med. 1992. 326:1464–1468.
4. Rothstein JD, Van Kammen M, Levey AI, Martin LJ, Kuncl RW. Selective loss of glial glutamate transporter GLT-1 in amyotrophic lateral sclerosis. Ann Neurol. 1995. 38:73–84.
5. Philips T, Robberecht W. Neuroinflammation in amyotrophic lateral sclerosis: role of glial activation in motor neuron disease. Lancet Neurol. 2011. 10:253–263.
6. Weydt P, Yuen EC, Ransom BR, Möller T. Increased cytotoxic potential of microglia from ALS-transgenic mice. Glia. 2004. 48:179–182.
7. Turner MR, Cagnin A, Turkheimer FE, Miller CC, Shaw CE, Brooks DJ, Leigh PN, Banati RB. Evidence of widespread cerebral microglial activation in amyotrophic lateral sclerosis: an [11C](R)-PK11195 positron emission tomography study. Neurobiol Dis. 2004. 15:601–609.
8. Henkel JS, Beers DR, Zhao W, Appel SH. Microglia in ALS: the good, the bad, and the resting. J Neuroimmune Pharmacol. 2009. 4:389–398.
9. Lasiene J, Yamanaka K. Glial cells in amyotrophic lateral sclerosis. Neurol Res Int. 2011. 2011:718987.
10. González-Scarano F, Baltuch G. Microglia as mediators of inflammatory and degenerative diseases. Annu Rev Neurosci. 1999. 22:219–240.
11. Tikka TM, Vartiainen NE, Goldsteins G, Oja SS, Andersen PM, Marklund SL, Koistinaho J. Minocycline prevents neurotoxicity induced by cerebrospinal fluid from patients with motor neurone disease. Brain. 2002. 125:722–731.
12. Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J. Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J Neurosci. 2001. 21:2580–2588.
13. Kojima M, Kangawa K. Ghrelin: structure and function. Physiol Rev. 2005. 85:495–522.
14. Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000. 141:4255–4261.
15. Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999. 402:656–660.
16. Peino R, Baldelli R, Rodriguez-Garcia J, Rodriguez-Segade S, Kojima M, Kangawa K, Arvat E, Ghigo E, Dieguez C, Casanueva FF. Ghrelin-induced growth hormone secretion in humans. Eur J Endocrinol. 2000. 143:R11–R14.
17. Chung H, Kim E, Lee DH, Seo S, Ju S, Lee D, Kim H, Park S. Ghrelin inhibits apoptosis in hypothalamic neuronal cells during oxygen-glucose deprivation. Endocrinology. 2007. 148:148–159.
18. Chung H, Seo S, Moon M, Park S. Phosphatidylinositol-3-kinase/Akt/glycogen synthase kinase-3β and ERK1/2 pathways mediate protective effects of acylated and unacylated ghrelin against oxygen-glucose deprivation-induced apoptosis in primary rat cortical neuronal cells. J Endocrinol. 2008. 198:511–521.
19. Hwang S, Moon M, Kim S, Hwang L, Ahn KJ, Park S. Neuroprotective effect of ghrelin is associated with decreased expression of prostate apoptosis response-4. Endocr J. 2009. 56:609–617.
20. Moon M, Kim HG, Hwang L, Seo JH, Kim S, Hwang S, Kim S, Lee D, Chung H, Oh MS, Lee KT, Park S. Neuroprotective effect of ghrelin in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson's disease by blocking microglial activation. Neurotox Res. 2009. 15:332–347.
21. Lim E, Lee S, Li E, Kim Y, Park S. Ghrelin protects spinal cord motoneurons against chronic glutamate-induced excitotoxicity via ERK1/2 and phosphatidylinositol-3-kinase/Akt/glycogen synthase kinase-3β pathways. Exp Neurol. 2011. 230:114–122.
22. Henkel JS, Engelhardt JI, Siklós L, Simpson EP, Kim SH, Pan T, Goodman JC, Siddique T, Beers DR, Appel SH. Presence of dendritic cells, MCP-1, and activated microglia/macrophages in amyotrophic lateral sclerosis spinal cord tissue. Ann Neurol. 2004. 55:221–235.
23. Lee J, Lim E, Kim Y, Li E, Park S. Ghrelin attenuates kainic acid-induced neuronal cell death in the mouse hippocampus. J Endocrinol. 2010. 205:263–270.
24. Lladó J, Haenggeli C, Maragakis NJ, Snyder EY, Rothstein JD. Neural stem cells protect against glutamate-induced excitotoxicity and promote survival of injured motor neurons through the secretion of neurotrophic factors. Mol Cell Neurosci. 2004. 27:322–331.
25. Rothstein JD, Jin L, Dykes-Hoberg M, Kuncl RW. Chronic inhibition of glutamate uptake produces a model of slow neurotoxicity. Proc Natl Acad Sci USA. 1993. 90:6591–6595.
26. Tolosa L, Caraballo-Miralles V, Olmos G, Lladó J. TNF-α potentiates glutamate-induced spinal cord motoneuron death via NF-κB. Mol Cell Neurosci. 2011. 46:176–186.
27. Lee JY, Chung H, Yoo YS, Oh YJ, Oh TH, Park S, Yune TY. Inhibition of apoptotic cell death by ghrelin improves functional recovery after spinal cord injury. Endocrinology. 2010. 151:3815–3826.
28. del Rio-Hortega P. Art and artifice in the science of histology. 1933. Histopathology. 1993. 22:515–525.
29. Huang CX, Yuan MJ, Huang H, Wu G, Liu Y, Yu SB, Li HT, Wang T. Ghrelin inhibits post-infarct myocardial remodeling and improves cardiac function through anti-inflammation effect. Peptides. 2009. 30:2286–2291.
30. Chow KB, Cheng CH, Wise H. Anti-inflammatory activity of ghrelin in human carotid artery cells. Inflammation. 2009. 32:402–409.
31. Theil MM, Miyake S, Mizuno M, Tomi C, Croxford JL, Hosoda H, Theil J, von Hörsten S, Yokote H, Chiba A, Lin Y, Oki S, Akamizu T, Kangawa K, Yamamura T. Suppression of experimental autoimmune encephalomyelitis by ghrelin. J Immunol. 2009. 183:2859–2866.
32. Erşahin M, Toklu HZ, Erzik C, Cetinel S, Akakin D, Velioğlu-Oğünç A, Tetik S, Ozdemir ZN, Sener G, Yeğen BC. The anti-inflammatory and neuroprotective effects of ghrelin in subarachnoid hemorrhage-induced oxidative brain damage in rats. J Neurotrauma. 2010. 27:1143–1155.
33. Andrews ZB, Erion D, Beiler R, Liu ZW, Abizaid A, Zigman J, Elsworth JD, Savitt JM, DiMarchi R, Tschoep M, Roth RH, Gao XB, Horvath TL. Ghrelin promotes and protects nigrostriatal dopamine function via a UCP2-dependent mitochondrial mechanism. J Neurosci. 2009. 29:14057–14065.
34. Jiang H, Li LJ, Wang J, Xie JX. Ghrelin antagonizes MPTP-induced neurotoxicity to the dopaminergic neurons in mouse substantia nigra. Exp Neurol. 2008. 212:532–537.
35. Obay BD, Tasdemir E, Tümer C, Bilgin HM, Sermet A. Antiepileptic effects of ghrelin on pentylenetetrazole-induced seizures in rats. Peptides. 2007. 28:1214–1219.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download