This article has been retracted. See "Retraction: A Novel Carbamoyloxy Arylalkanoyl Arylpiperazine Compound (SKL-NP) Inhibits Hyperpolarization-Activated Cyclic Nucleotide-Gated (HCN) Channel Currents in Rat Dorsal Root Ganglion Neurons" in Volume 16 on page 367.
Abstract
In this study, we determined mode of action of a novel carbamoyloxy arylalkanoyl arylpiperazine compound (SKL-NP) on hyperpolarization-activated cyclic nucleotide-gated (HCN) channel currents (Ih) that plays important roles in neuropathic pain. In small or medium-sized dorsal root ganglion (DRG) neurons (<40 µm in diameter) exhibiting tonic firing and prominent Ih, SKL-NP inhibited Ih and spike firings in a concentration dependent manner (IC50=7.85 µM). SKL-NP-induced inhibition of Ih was blocked by pretreatment of pertussis toxin (PTX) and N-ethylmaleimide (NEM) as well as 8-Br-cAMP, a membrane permeable cAMP analogue. These results suggest that SKL-NP modulates Ih in indirect manner by the activation of a Gi-protein coupled receptor that decreases intracellular cAMP concentration. Taken together, SKL-NP has the inhibitory effect on HCN channel currents (Ih) in DRG neurons of rats.
The hyperpolarization-activated cyclic nucleotide-gated (HCN) channels, expressed in many excitable cells including cardiac and neural cells, play physiological roles in cellular excitability by contributing to generation and modulation of action potential firings [1]. HCN family comprises four members (HCN1-4) [2], and it has been identified that their subtype expression patterns are different among separate populations of sensory neurons. Ih, the current produced by HCN channels, is expressed differentially in various subpopulations of rat sensory neurons, being more prominent in Aβ-type sensory neurons than C- and Aδ-type sensory neurons [3].
Peripheral nerve injury often produces neuropathic pain, characterized by spontaneous pain, thermal hyperalgesia and mechanical allodynia [4]. Spontaneous ectopic discharges of injured primary sensory neurons, particularly in medium- and large-sized neurons are associated with HCN channels, which is believed to be one of the major peripheral mechanisms involved in the pathogenesis of neuropathic pain [3]. Consistent with the previous studies, our group also found that Ih is more preferentially important for mechanical allodynia than thermal hyperalgesia [5].
A specific blocker of Ih, ZD7288, reverses both pain behavior and the spontaneous discharges in injured nerve fibers [6], indicating that pharmacological blockade of Ih could have a therapeutic potential for the treatment of neuropathic pain, especially mechanical allodynia. Our recent work also showed that Ih represents a valid target for novel analgesics useful for reversing mechanical allodynia of neuropathic pain [5].
In this study, we investigate mode of action of a novel analgesics, carbamoyloxy arylalkanoyl arylpiperazine compound (R-Carbamic acid 3-[4-(3,4-dimethoxy-phenyl)-piperazin-1-yl]-3-oxo-1-phenyl-propyl ester, Fig. 1, molecular weight 509.5, SKL-NP, SK biopharmaceuticals, Inc., Korea), by using whole-cell patch clamp recordings from rat DRG neurons.
Our initial work was focused on exploration of the structurally novel arylpiperazine as analgesics, because its elements are most frequently served as pharmacophores in central nervous system field. During the structural modification, we identified SKL-NP as having an analgesic effect of pain models, but its analgesic mechanism was not elucidated yet. Chemical structure of SKL-NP is shown in Fig. 1. Family patent applications of this compound were filed in several countries including Korea (PCT/KR2008/002470), US (US2011/0195963 A1), EU, Brazil, Canada, China, Japan and so on.
All surgical and experimental procedures complied with guidelines from the Institutional Animal Care and Use Committee (IACUC) at the School of Dentistry, Seoul National University. DRG neurons from 2- to 7-day-old neonatal rats (OrientBio, Sungnam, Korea) were prepared as previously described [7]. Briefly, DRG neurons taken from L3-5 DRG were prepared in 4℃ HBSS (Life Technologies, Carlsbad CA, USA) and incubated in 3 ml DMEM (Invitrogen, Carlsbad CA, USA) containing collagenase (5 mg/ml; Sigma-Aldrich, St. Louis, MO, USA) and dispase (1 mg/ml; Sigma-Aldrich) then in 2 ml DMEM of 0.25% trypsin (Sigma-Aldrich) at 37℃ for 8 min. The cells were washed in DMEM and triturated with a flame-polished Pasteur pipette to separate cells and remove processes, and subsequently centrifuged (750 RPM, 5 min), re-suspended and placed on poly-D-lysine (Sigma-Aldrich)-coated glass cover-slips (12 mm in diameter). The cells were maintained at 37℃ in 95% O2/5% CO2 incubator.
Whole-cell recording were made using patch electrodes (4~6 MΩ) pulled from borosilicate glass on a Brown-Flaming P-97 horizontal micropipette puller (Sutter Instrument, USA). Voltage- and current-clamp experiments were performed using an HEKA EPC9 amplifier and digitized using ITC16 and Pulse v8.54 software (both from HEKA, Germany). Signals were filtered at 1 kHz and sampled at 3 kHz. Series resistance was typically less than 20 MΩ and was compensated by about 70~80%. Data were analyzed using Origin6.0 software (Microcal Software Inc., USA). All recordings were performed at room temperature (21~23℃).
Extracellular solution contained (in mM): 140 NaCl, 2 CaCl2, 1 MgCl2, 5 KCl, 10 HEPES, 10 D-Glucose, adjusted to pH 7.4 with NaOH, 290~310 mOsm. In experiments, BaCl2 (500 µM/l) was used to eliminate inward rectifier K+ currents. Pipettes were filled with an intracellular solution containing (in mM): 136 K-gluconate, 10 NaCl, 1 MgCl2, 10 EGTA, 2 Mg-ATP, 0.1 Na-GTP adjusted to pH 7.4 with KOH, 290~310 mOsm.
Ih were elicited every 15 sec by a 1 s pulse from a holding potential of -50 mV to a command potential of -100 mV. SKL-NP was bath-applied after obtaining stable Ih for 5 min. Recorded Ih was normalized for pulled data analysis. IC50 was determined as concentration of SKL-NP that would inhibit 50% of the maximum amplitude of Ih based on curve drawn by Origin software. In current-clamp experiments, potential change or action potential firing was elicited by either hyperpolarizing or depolarizing current pulses.
In our previous study, we found that the mechanical allodynia in neuropathic pain could be associated with Ih that contributes ectopic action potential firings in sensory neurons [5]. Thus, we investigated whether SKL-NP affects the firing pattern in small or medium-sized DRG neurons (<40 µm in diameter), which were characterized by tonic discharge of action potentials and prominent Ih. As shown in Fig. 2Aa, tonic firing and Ih-induced sag in DRG neuron were mostly decreased by 10 µM SKL-NP (58.2±10.4%, n=7, p<0.05). In addition, Ih was blocked by SKL-NP (51.6±3.5%, n=7, p<0.05, Fig. 2Ab). However, tonic firing pattern in DRG neurons with no discernible Ih was not altered by SKL-NP (n=4, p>0.05, Fig. 2B).
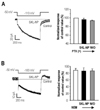
Next, we determined the concentration-dependent effect of SKL-NP on Ih. Maximal inhibitory effect of SKL-NP was 52.7% inhibition of Ih at 50 µM, and IC50 was 7.85 µM (Fig. 3A). These results suggest that SKL-NP reduced the excitability of small and medium-sized DRG neurons by inhibiting Ih in a cell-specific and dose-dependent manner. In addition, inhibitory effect of SKL-NP on Ih showed a delayed response in an irreversible manner (Fig. 3B).
Because HCN channels are regulated by cyclic nucleotide in addition to hyperpolarization, we next examined whether the modulating mechanism of SKL-NP effect on Ih is associated with Gi-protein. The pretreatment of pertussis toxin (PTX, 4 µg/ml) and NEM (50 µM) [8] abolished the inhibitory effect of SKL-NP on Ih during application of SKL-NP and at 5 min after application of SKL-NP (96.4±5.3% and 94.5±0.5%, n=7, p>0.05, Fig. 4A). Thus, SKL-NP inhibits Ih in DRG neurons via PTX-sensitive Gi-protein activation. Another Gi-protein inhibitor, NEM, which slightly decreased the amplitude of Ih, (88.7±5.7%, n=4, p>0.05, Fig. 4B), also blocked the SKL-NP effect on Ih (86.0±9.8%, n=4, p>0.05). These results indicate that DRG neurons expressing a Gi-protein coupled receptor (GPCR) may be sensitive to SKL-NP, and their excitability is inhibited by SKL-NP via indirect pathway.
Next, we examined the Gi-protein downstream pathway (i.e. the decrease of cAMP via inhibition of adenylyl cyclase) with the pretreatment of 8-Br-cAMP (200 µM), a membrane permeable cAMP analog, for 10 min before application of SKL-NP. In DRG neurons tested (n=4), 8-Br-cAMP application induced the fast activation of Ih, without affecting the amplitude of Ih (100.3±7.2%, n=4, p>0.05). SKL-NP-induced Ih inhibition was not observed by the pretreatment of 8-Br-cAMP (91.8±18.7%, n=4, p>0.05), indicating that SKL-NP effect was associated with decrease of intracellular cAMP concentration (Fig. 5).
Although neuropathic pain is most intractable pain found in the clinic, current therapeutic treatments and analgesic agents are yet to be successful. In the present study, we explored the mode of action of a novel compound (SKL-NP) that produces anti-allodynic effects. Given the critical role of Ih in mechanical allodynia [9,10], we examined the effect of SKL-NP on Ih and found that SKL-NP inhibits Ih in a dose-dependent manner in distinct subpopulations of DRG neurons; tonic firing neurons by depolarizing current injection. SKL-NP-induced Ih inhibition is likely to be indirectly mediated via the activation of a Gi-protein coupled receptor that results in the decrease of intracellular cAMP, rather than via Ih inhibition with direct binding to HCN channel.
Ih was observed in the majority (n=13/17) of neurons with tonic firings, which was readily blocked by 1 mM Cs+, a well-known Ih blocker, suggesting the association of Ih activation with the generation of tonic discharges. In sensory neurons, Ih results from the HCN channel family which comprises four members (HCN1-4) with different activation kinetics with order of HCN1>HCN2>HCN3>HCN4, and sensitivity to cAMP; HCN2 and HCN4 are more sensitive to cAMP than HCN1 and HCN3 [1]. Given that neurons tested in this work showed sensitivity to 8Br-cAMP and HCN4 was poorly expressed in all DRG neurons [11], and HCN2 is preferentially expressed in small neurons [12], we assumed that HCN2 subtype could play a major functional role among HCN subtypes in our experimental setup.
Numerous GPCRs have been reported to modulate Ih [3]. For example, morphine, an agonist for the Gi/o-coupled µ-opioid receptor, decreased Ih by lowering cAMP; this has been speculated to be one of the mechanisms by which morphine reduces primary afferent excitability and relieves pain [13]. Thus, we tested whether Gi/o-protein is involved in SKL-NP action on Ih. Two pharmacological blockers of Gi/o-protein, PTX and NEM [8], reversed SKL-NP-induced Ih inhibition, indicating that Gi/o-protein is involved in SKL-NP action. Given that cAMP is the key allostreric modulator for HCN channels, we also determined whether SKL-NP-induced Ih inhibition would be influenced by intracellular cAMP. We observed that the inhibitory effect of SKL-NP on Ih was occluded by the pretreatment of 8Br-cAMP; in this condition, intracellular cAMP concentration did not change by regulating either Gi-protein or adenylyl cyclase. These results suggest that SKL-NP effect may be attributable to decrease of intracellular cAMP concentration that results from the activation of a certain type of GPCR. Recently, we showed that eugenol might produce direct inhibition of Ih rather than indirect action through Gi/o-protein [5]. However, SKL-NP, unlike eugenol, seems to inhibit Ih via decreasing cAMP following preceding action on a GPCR. The GPCR targeted by SKL-NP remains to be determined.
Several studies revealed that Ih has an important role in mechanical allodynia in neuropathic pain conditions [9,10]. Chaplan et al. [6] suggested the importance of HCN channels in both mechanical pain and spontaneous firings originating in the damaged DRG. Consistent with the previous studies, we recently found that Ih is more preferentially important for mechanical allodynia than thermal hyperalgesia, and mechanical allodynia could be targeted by a specific antagonist for HCN channels [5].
From this study, we found that SKL-NP has anti-allodynic effect via inhibition of Ih in DRG neurons. Given that Ih is associated not only with the generation of tonic discharge, but also ectopic spontaneous discharges of DRG neurons following peripheral nerve injury, SKL-NP might be therapeutically applicable for the treatment of mechanical allodynia in neuropathic patients. Considering a recent study suggesting a pivotal role of HCN2 in neuropathic pain [12], HCN2 selective blockers may inhibit pain associated with nerve injury with less side-effect on heart, where HCN4 is highly expressed and plays a predominant role in cardiac pacemaker tissue.
Figures and Tables
Fig. 1
Structure of a novel carbamoyloxy arylalkanoyl arylpiperazine compound (R-Carbamic acid 3-[4-(3,4-dimethoxy-phenyl)-piperazin-1-yl]-3-oxo-1-phenyl-propyl ester, molecular weight 388.27, SKL-NP).
Fig. 2
The effects of SKL-NP on Ih in rat DRG neurons. (A) (a) Representative trace of firing pattern elicited by a 2 s current injection (500 pA) before and during exposure to SKL-NP (10 µM). SKL-NP abolished tonic firing. Injection of a hyperpolarizing current (-100 pA) elicited a sag in the voltage trace (bottom trace), which was abolished by application of SKL-NP (10 µM). (b) Representative trace of Ih currents, which was inhibited by SKL-NP (10 µM). (c) Summary of firing rates (white columns) and amplitude of Ih (black columns) under influence of SKL-NP (n=7, *p<0.05). (B) Tonic firing of neurons, which did not have discernable Ih, was not modified by application of SKL-NP (n=4, p>0.05).
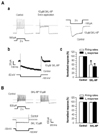
Fig. 3
(A) Concentration-response curve of SKL-NP effects on Ih. SKL-NP inhibited Ih, and spike firings in concentration dependent manner. (B) An irreversible action of SKL-NP effects on Ih. Inhibitory effect of SKL-NP showed a delayed response in a irreversible manner. After washout of SKL-NP, Ih did not recover to control level (SKL-NP 10 and 50 µM, *p<0.05).

Fig. 4
Signaling pathways involved in SKL-NP-induced Ih inhibition. (A) PTX abolished the inhibitory effects of SKL-NP on Ih (n=7). Superimposed Ih traces before and after application of SKL-NP following PTX pretreatment. (B) Another Gi-protein inhibitor, NEM, also blocked the inhibitory effects of SKL-NP on Ih during and after SKL-NP application (n=4, p>0.05). Superimposed Ih traces before and after application of SKL-NP following NEM pretreatment.
ACKNOWLEDGEMENTS
This work was supported by the research fund of Hanyang University (HY-201000000000283).
References
1. Momin A, Cadiou H, Mason A, McNaughton PA. Role of the hyperpolarization-activated current Ih in somatosensory neurons. J Physiol. 2008. 586:5911–5929.
2. Wahl-Schott C, Biel M. HCN channels: structure, cellular regulation and physiological function. Cell Mol Life Sci. 2009. 66:470–494.
3. Wickenden AD, Maher MP, Chaplan SR. HCN pacemaker channels and pain: a drug discovery perspective. Curr Pharm Des. 2009. 15:2149–2168.
4. Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron. 2006. 52:77–92.
5. Yeon KY, Chung G, Kim YH, Hwang JH, Davies AJ, Park MK, Ahn DK, Kim JS, Jung SJ, Oh SB. Eugenol reverses mechanical allodynia after peripheral nerve injury by inhibiting hyperpolarization-activated cyclic nucleotide-gated (HCN) channels. Pain. 2011. 152:2108–2116.
6. Chaplan SR, Guo HQ, Lee DH, Luo L, Liu C, Kuei C, Velumian AA, Butler MP, Brown SM, Dubin AE. Neuronal hyperpolarization-activated pacemaker channels drive neuropathic pain. J Neurosci. 2003. 23:1169–1178.
7. Binshtok AM, Bean BP, Woolf CJ. Inhibition of nociceptors by TRPV1-mediated entry of impermeant sodium channel blockers. Nature. 2007. 449:607–610.
8. Shapiro MS, Wollmuth LP, Hille B. Modulation of Ca2+ channels by PTX-sensitive G-proteins is blocked by N-ethylmaleimide in rat sympathetic neurons. J Neurosci. 1994. 14:7109–7116.
9. Dunlop J, Vasilyev D, Lu P, Cummons T, Bowlby MR. Hyperpolarization-activated cyclic nucleotide-gated (HCN) channels and pain. Curr Pharm Des. 2009. 15:1767–1772.
10. Jiang YQ, Sun Q, Tu HY, Wan Y. Characteristics of HCN channels and their participation in neuropathic pain. Neurochem Res. 2008. 33:1979–1989.
11. Kouranova EV, Strassle BW, Ring RH, Bowlby MR, Vasilyev DV. Hyperpolarization-activated cyclic nucleotide-gated channel mRNA and protein expression in large versus small diameter dorsal root ganglion neurons: correlation with hyperpolarization-activated current gating. Neuroscience. 2008. 153:1008–1019.
12. Emery EC, Young GT, Berrocoso EM, Chen L, McNaughton PA. HCN2 ion channels play a central role in inflammatory and neuropathic pain. Science. 2011. 333:1462–1466.
13. Ingram SL, Williams JT. Opioid inhibition of Ih via adenylyl cyclase. Neuron. 1994. 13:179–186.




