Abstract
We evaluated the role of Tat-mediated p66shc transduction on the activation of endothelial nitric oxide synthase in cultured mouse endothelial cells. To construct the Tat-p66shc fusion protein, human full length p66shc cDNA was fused with the Tat-protein transduction domain. Transduction of TAT-p66shc showed a concentration- and time-dependent manner in endothelial cells. Tat-mediated p66shc transduction showed increased hydrogen peroxide and superoxide production, compared with Tat-p66shc (S/A), serine 36 residue mutant of p66shc. Tat-mediated p66shc transduction decreased endothelial nitric oxide synthase phosphorylation in endothelial cells. Furthermore, Tat-mediated p66shc transduction augmented TNF-α-induced p38 MAPK phosphorylation in endothelial cells. These results suggest that Tat-mediated p66shc transduction efficiently inhibited endothelial nitric oxide synthase phosphorylation in endothelial cells.
Shc is an adaptor protein containing a C-terminal src homology collagen domain-2. The mammalian adaptor protein ShcA has three isoforms with relative molecular masses of 46, 52, and 66 kDa. p66shc possesses an N-terminal collagen-homology domain that is not present in p46shc or p52shc. p66shc is phosphorylated on serine 36 within the N-terminal domain in response to oxidative stress [1]. Several studies have shown that endothelial dysfunction by oxidative stress is central in the pathogenesis of vascular dysfunction and atherogenesis [2-4]. Recently, it was reported that the p66shc(-/-) mouse is a unique genetic model for increased resistance to oxidative stress and prolonged life span in mammals [5]. Therefore, p66shc might represent a molecular target for therapies against vascular diseases.
The phosphorylation of endothelial nitric oxide synthase (eNOS) in endothelial cells is pivotal in the defense against vascular inflammatory diseases [6] and atherosclerosis [7]. Elucidation of the role of p66shc adaptor protein on eNOS activity will contribute to our understanding of vasomotor physiology and the pathophysiology of endothelial dysfunction and could provide insights for new therapies, particularly in hypertension and atherosclerosis. Genetic deletion of p66shc prevents endothelial dysfunction [8,9]. RNAi-mediated down-regulation of endogenous p66shc leads to the activation of endothelial nitric oxide synthase at serine 1177 [10]. Furthermore, p66shc phosphorylation is closely related to oxidative stress in endothelial cells [11] or hypertensive animals such as coarctation of aorta [12].
Direct intracellular delivery of proteins has been difficult to achieve due primarily to the plasma membrane barrier, which effectively prevents macromolecule uptake by limiting passive entry. One approach to circumvent these problems is to use HIV TAT-mediated protein transduction [13]. Protein transduction domains (PTD) offer an exciting therapeutic opportunity to treat many diseases such as vascular inflammation [14,15]. Using a PTD-fused protein might also help us understand target protein signaling, as its cellular transduction is rapid.
Our aim was to evaluate the potential usefulness of the Tat-p66shc fusion protein on the eNOS phosphorylation in endothelial cells. We investigated the transduction of the full length human Tat-p66shc fusion protein in endothelial cells and the resulting biological activity on eNOS phosphorylation in cultured endothelial cells.
Mouse MS-1 endothelial cells were purchased from the American Type Culture Collection (Manassas, VA, USA). Cells were grown in Dulbecco's-modified Eagle medium (DMEM) with 10% fetal bovine serum, 10 U/ml penicillin, and 10 µg/ml streptomycin. Antibodies to Shc (SC-1695, Santa Cruz Biotechnology, Santa Cruz, CA, USA), phospho-ser36-p66shc (CN566807, Calbiochem, La Jolla, CA, USA), eNOS (SC-654, Santa Cruz Biotechnology, Santa Cruz, CA, USA), phospho-ser1199-eNOS (#9571, Cell Signaling Technology, Danvers, MA, USA), and β-actin (SC-1616, Santa Cruz Biotechnology, Santa Cruz, CA, USA) were used. Horseradish peroxidase (HRP)-labeled anti-rabbit antibody was obtained from Pierce biotechnology (Rockford, IL USA). Ni-nitrilotriacetic acid Sepharose was purchased from Qiagen (Valencia, CA, USA). Isopropyl-β-thiogalactoside (IPTG) was purchased from USB (Cleveland, OH, USA).
Tat-p66shc was generated by inserting p66shc cDNA into pTAT-2.1. The human p66shc was isolated from Xpress-tagged p66shc cDNA in pcDNA3/1/His A (Invitrogen, Carlsbad, CA, USA) [10] by PCR using the following two primers; the sense primer was 5'-CGG GAT CCC GGA ATT CGG CTT ATG GAT CTC C-3' (containing an EcoRI restriction site), and the antisense primer was 5'-CGA AGC TTT CAC AGT TTC CGC TCC ACA GG-3' (containing a HindIII restriction site). After digesting with EcoRI and Hind III, the full length p66shc constructs were cloned into the pTAT bacterial expression vector (pTAT-2.1, kindly donated by Steven Dowdy), which contains a six-histidine tag, for easy purification. p66shc (S36A) cDNA was kindly donated by Kaikobad Irani (Pittsburgh University). pTAT-p66shc plasmids were then transformed into the BL21 (DE3) strain of E. coli. Following 4 hours of induction with IPTG, the cells were sonicated in buffer Z (8 M urea, 100 mM NaCl, 20 mM HEPES) and recombinant proteins were purified on a Ni-NTA agarose column (Qiagen). After washing, TAT-p66shc was eluted using 250 mM imidazole containing Buffer Z followed by desalting on a PD-10 column (Amersham Pharmacia Biotech, Piscataway, NJ, USA) in PBS. The eluate was frozen in 10% glycerol at -80℃. p66shc protein as control protein was purified from pET28b/p66shc expression vector with same protocol for Tat-p66shc.
MS-1 cells were grown in DMEM at 37℃ in humidified 95% air/5% CO2. The cells were grown to confluence on a 6-well plate and then treated with various concentrations of Tat-p66shc for the indicated times. Cell extracts were prepared for Western blot analysis.
MS-1 cells were harvested with 100 µl of lysis buffer containing 20 mM Tris-Cl, pH 7.5, 100 mM NaCl, 2 mM EDTA, 2 mM EGTA, 1 mM Na3VO3, 1 mM beta-glycerophosphate, 4 mM Na pyrophosphate, 5 mM NaF, 1% Triton X-100, and a protease inhibitor cocktail. The lysate was centrifuged at 12,000 rpm for 20 min, and the supernatant was collected. Protein (30 µg) was separated by 10% SDS-PAGE and was electrotransfered onto nitrocellulose membranes. After blocking with 5% skim milk for 2 h at room temperature, blots were incubated overnight at 4℃ with specific primary antibody (1:1,000) and subsequently with horseradish peroxidase (HRP)-conjugated secondary antibody. Blots were developed for visualization using an enhanced chemiluminescence detection kit (Pierce). Cells were serum starved for 18 h in some experiments to reduce basal phosphorylation of specific proteins.
Intracellular hydrogen peroxide production was detected using the peroxide-sensitive fluorophore 2',7'-dichlorodihydrofluorescein diacetate (DCF-DA), as described previously [6,16]. The cells were cultured in chamber slides (2×105 cells/well) (Nalgen Nunc International, Rochester, NY, USA) to detect DCF-DA fluorescence. The cells were rinsed three times and incubated for 30 min with 10 µM DCF-DA at 37℃ in Krebs-HEPES buffer and Hanks buffered salts solution, respectively. The absolute fluorescence of 20~25 random cells was quantified using MetaMorph software (Molecular Devices, Sunnyvale, CA, USA). Superoxide production was assayed in the cultured endothelial cells using lucigenin chemiluminescence. In briefly, darkadapted lucigenin solution (5 µM) was prepared in aerated Krebs-Hepes buffer. Cells were immersed in lucigenin solution and chemiluminescence detected with a Monolight luminometer. The chemiluminescence signal was integrated over 2 minutes.
First, we constructed the Tat-p66shc fusion protein using recombinant DNA technology. The Tat-p66shc expression vector contained a consecutive cDNA sequence encoding human p66shc, the Tat-PTD (RKKRRQRRR), and six histidine residues at the amino-terminus (Fig. 1). Tat-p66shc proteins were serially eluted with the treatment of 250 mM imidazole containing buffer Z. The purified Tat-p66shc proteins were confirmed by Commassie blue staining, which showed a molecular weight of approximately 70.5 kDa (Fig. 1).
Purified Tat-p66shc (30 nM) was added to cultured endothelial cells for various incubation times to evaluate the transduction ability in cultured endothelial cells. After the incubation, the cells were harvested and the change in transduced p66shc was analyzed with Western blotting using the anti-Shc antibody. As shown in Fig. 2A, the Tat-p66shc fusion protein was detected in the cell lysates within 15 min and its transduction reached a maximum at 3 h. A significant level of transduced Tat-p66shc was present in the cells at 24 h (Fig. 2A). Moreover, Tat-p66shc fusion proteins were transduced into the cells in a dose-dependent manner in the range of 1~100 nM (Fig. 2B).
We next studied whether transduced Tat-p66shc affected hydrogen peroxide and superoxide production in cultured endothelial cells. Endothelial cells were incubated with Tat-p66shc, Tat-p66shc (S/A), p66shc, and Tat-GFP as a control for 3 h. After the incubation, hydrogen peroxide and superoxide production was measured in the endothelial cells using DCF-DA and lucigenin chemiluminescence. As shown in Fig. 3, Tat-p66shc (30 nM) for 3 h significantly elevated intracellular hydrogen peroxide compared with Tat-GFP and p66shc as control in endothelial cells. Also, the Tat-p66shc incubation (30 nM) increased superoxide production, as accessed by lucigenin chemiluminescence. However, Tat-p66shc-mediated ROS productions were greater than that of Tat-p66shc (S/A), suggesting important role of serine 36 residue of p66shc in ROS productions in endothelial cells.
To understand the acute effect of p66shc on eNOS activity, we examined the effect of Tat-p66shc transduction on eNOS phosphorylation in cultured endothelial cells. In comparison to Tat-GFP treated cells, Tat-p66shc transduction did not affect eNOS protein expression; however, it decreased eNOS phosphorylation at the serine 1177 residue (Fig. 4A).
Finally, we investigated the role of Tat-p66shc protein transduction on eNOS phosphorylation in tumor necrosis factor (TNF)-α-stimulated endothelial cells. As shown in Fig. 4B, TNF-α increased p38 MAPK and eNOS phosphorylation. In contrast, Tat-p66shc protein transduction (30 nM for 3 h) augmented TNF-α-induced p38 MAPK phosphorylation and abrogated TNF-α-induced eNOS phosphorylation in endothelial cells.
The present study demonstrated that transduced Tat-p66shc fusion protein increased intracellular ROS production and inhibited eNOS phosphorylation in vascular endothelial cells.
Protein transduction domains (PTD) offer an exciting therapeutic opportunity to treat many diseases such as vascular inflammation. Using a PTD-fused protein might also help us understand target protein signaling. In the present study, we generated Tat-p66shc protein with denature condition to see the biological effect in endothelial cells. Tat-p66shc fusion protein was detected in the cell lysates within 15 min and its transduction reached a maximum at 3 h and its transduction was shown in dose-dependent manner. Our data showed the cellular transduction of purified Tat-p66shc protein in cultured endothelial cells. In particular, Tat-PTD (RKKRRQRRR) helped to efficiently translocate p66shc across plasma membrane [17]. Due to its rapid transduction into the cells, the Tat-p66shc protein is applicable to understanding p66shc signaling and avoids the need for genetic manipulation such as adenoviral DNA or plasmid transfection.
P66shc knockout mice showed decreased vascular production of superoxide, which contributes to increase NO availability [18]. Cells deficient in p66shc have reduced susceptibility to oxidative stress, and less generation of reactive oxygen species, so the reduction of p66shc suppresses oxidative damage [19]. We next studied whether transduced Tat-p66shc affected hydrogen peroxide and superoxide production in cultured endothelial cells. Tat-p66shc incubation increased superoxide production, suggesting that direct transduction of p66shc using Tat-p66shc modulated intracellular reactive oxygen species in endothelial cells. In the present study, we confirmed that the role of serine 36 in the p66shc has been characterized. Tat-mediated p66shc (serine 36 to alanine mutation) transduction resulted in few increase of ROS production than wild type of p66shc. It is suggested that Tat-p66shc-mediated ROS production has been implicated in phosphorylating p66shc on serine 36.
Abnormal endothelial nitric oxide production is closely related with the pathogenesis of atherosclerosis and hypertension [20]. Modulation of endothelial nitric oxide synthase (eNOS) activation is a target protein for treating cardiovascular disorder [21-23]. The p66shc adaptor protein has an inhibitory role in eNOS activity [24] and endothelial vasomotor function [10]. However, the acute response of p66shc on eNOS activity is unknown. To understand the acute effect of p66shc on eNOS activity, we examined the effect of Tat-p66shc transduction on eNOS phosphorylation in cultured endothelial cells. TNF-α treatment led to p38 MAPK phosphorylation in endothelial cells, suggesting that p38 MAPK signaling is involved in TNF-α-induced endothelial cell activation [25,26]. However, the effect of p66shc transduction on p38 MAPK in TNF-α-stimulated endothelial cells remains unknown. Our data showed that p66shc transduction augmented p38 MAPK activation in endothelial cells, suggesting that Tat-p66shc transduction increased p38 MAPK activation via increase in reactive oxygen species in endothelial cells.
Previous studies have demonstrated that TNF-α-induced p38 MAPK activation decreases eNOS activation, whereas p38 MAPK inhibitor treatment restores eNOS activity in endothelial cells [27,28]. Furthermore, activation of the p38 MAPK signaling pathway is implicated in the downregulation of eNOS promoter activity [29]. In the present study, Tat-p66shc-mediated suppression of eNOS phosphorylation persisted even in the presence of TNF-α, suggesting that Tat-p66shc-mediated suppression of eNOS phosphorylation is regulated by p38 MAPK activation.
Taken together, these data show that the Tat-p66shc protein was efficiently transduced into cultured endothelial cells and suppressed eNOS phosphorylation in endothelial cells, suggesting Tat-mediated p66shc transduction may be useful for suppressing eNOS activation in vascular pathogenesis.
Figures and Tables
Fig. 1
Schematic diagrams, Commassie blue staining and purification of Tat-p66shc fusion proteins. (A) The human p66shc coding frame is presented with six histidines and the Tat PTD domain (RKKRRQRRR). Diagram of the p66shc fusion protein was represented on the vector. (B) Commassie blue staining of the Tat-p66shc protein. Lane 1 (L) : Escherichia coli BL21 (DE3) lysates after ITPG induction, Lane 2 (W): Eluate with 10 mM imidazole-containing buffer Z, Lanes 3, 4. The serial eluates with 250 mM imidazolecontaining buffer Z. (C) Purification steps for Tat-p66shc fusion proteins.
Fig. 2
Transduction of Tat-p66shc proteins in cultured MS-1 endothelial cells. (A) Time-dependent cellular uptake of Tat-p66shc. Purified Tat-p66shc (30 nM) was added to the culture medium for the indicated times, and then cell lysates were subjected to Western blot analysis with anti-SHC antibody. Densitometric data analysis is plotted at the bottom. Expression levels are shown as % expression to maximum. Each bar shows the mean±S.E. (n=3). (B) Dose-dependent transduction of Tat-p66shc. Purified Tat-p66shc proteins were incubated with the indicated concentration for 3 h. Densitometric analysis data is plotted at the bottom. Expression levels are shown as % of β-actin. Each bar shows the mean±S.E. (n=3). Arrows at 66, 52, and 46 indicate the endogenous SHC protein expression. Arrow at 70.9 indicates the Tat-p66shc protein.
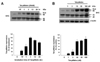
Fig. 3
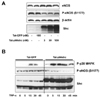
Tat-p66shc transduction increased hydrogen peroxide and superoxide production in culture MS-1 endothelial cells. (A) Effect of Tat-p66shc on hydrogen peroxide production. Intracellular hydrogen peroxide production was evaluated using the peroxide-sensitive fluorophore 2',7'-dichlorodihydrofluorescein diacetate (DCF-DA). Control: p66shc protein alone, Tat-GFP (30 nM), Tat-p66shc (30 nM), Tat-p66shc S/A (30 nM). (B) Effect of Tat-p66shc on superoxide production. Intracellular superoxide production was evaluated using lucigenin chemiluminescence. Superoxide production levels are expressed as relative luminescence units, RLU/105 cells. Control: p66shc protein alone, Tat-GFP (30 nM), Tat-p66shc (30 nM), Tat-p66shc S/A (30 nM). Each bar shows the mean±S.E. (n=5). NS, not significant, *p<0.05, #p<0.05 versus p66shc.

Fig. 4
Tat-p66shc transduction decreased endothelial nitric oxide synthase (eNOS) phosphorylation in culture MS-1 endothelial cells. (A) Effect of Tat-p66shc on basal eNOS phosphorylation. Tat-p66shc and Tat-GFP were incubated for 3 h with endothelial cells at the indicated concentrations, and then cell lysates were subjected to Western blot analysis. Note: Cells were not serum starved to detect basal eNOS phosphorylation. (B) Effect of Tat-p66shc on eNOS phosphorylation in the tumor necrosis factor (TNF)-α-stimulated endothelial cells for 0~45 min. Tat-p66shc (30 nM) and Tat-GFP (30 nM) were incubated for 3 h, and then TNF-α was treated for the indicated times. The cells were serum starved for 18 h to reduce basal p38 MAPK and eNOS phosphorylation. This figure shows a representative experiment out of 3 made.
ACKNOWLEDGEMENTS
We thank Dr. Steven Dowdy (University of California, San Diego, USA) for providing the Tat expression vector. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (2011-0006231, 2011-0016797 to B.H.J and 2011-0014483 to S.K.L).
References
1. Migliaccio E, Mele S, Salcini AE, Pelicci G, Lai KM, Superti-Furga G, Pawson T, Di Fiore PP, Lanfrancone L, Pelicci PG. Opposite effects of the p52shc/p46shc and p66shc splicing isoforms on the EGF receptor-MAP kinase-fos signalling pathway. EMBO J. 1997. 16:706–716.
2. Stocker R, Keaney JF Jr. Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004. 84:1381–1478.
3. Molavi B, Mehta JL. Oxidative stress in cardiovascular disease: molecular basis of its deleterious effects, its detection, and therapeutic considerations. Curr Opin Cardiol. 2004. 19:488–493.
4. Madamanchi NR, Vendrov A, Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol. 2005. 25:29–38.
5. Napoli C, Martin-Padura I, de Nigris F, Giorgio M, Mansueto G, Somma P, Condorelli M, Sica G, De Rosa G, Pelicci P. Deletion of the p66Shc longevity gene reduces systemic and tissue oxidative stress, vascular cell apoptosis, and early atherogenesis in mice fed a high-fat diet. Proc Natl Acad Sci USA. 2003. 100:2112–2116.
6. Kim CS, Son SJ, Kim EK, Kim SN, Yoo DG, Kim HS, Ryoo SW, Lee SD, Irani K, Jeon BH. Apurinic/apyrimidinic endonuclease1/redox factor-1 inhibits monocyte adhesion in endothelial cells. Cardiovasc Res. 2006. 69:520–526.
7. Kawashima S. Malfunction of vascular control in lifestyle-related diseases: endothelial nitric oxide (NO) synthase/NO system in atherosclerosis. J Pharmacol Sci. 2004. 96:411–419.
8. Martin-Padura I, de Nigris F, Migliaccio E, Mansueto G, Minardi S, Rienzo M, Lerman LO, Stendardo M, Giorgio M, De Rosa G, Pelicci PG, Napoli C. p66Shc deletion confers vascular protection in advanced atherosclerosis in hypercholesterolemic apolipoprotein E knockout mice. Endothelium. 2008. 15:276–287.
9. Camici GG, Schiavoni M, Francia P, Bachschmid M, Martin-Padura I, Hersberger M, Tanner FC, Pelicci P, Volpe M, Anversa P, Lüscher TF, Cosentino F. Genetic deletion of p66(Shc) adaptor protein prevents hyperglycemia-induced endothelial dysfunction and oxidative stress. Proc Natl Acad Sci USA. 2007. 104:5217–5222.
10. Yamamori T, White AR, Mattagajasingh I, Khanday FA, Haile A, Qi B, Jeon BH, Bugayenko A, Kasuno K, Berkowitz DE, Irani K. P66shc regulates endothelial NO production and endothelium-dependent vasorelaxation: implications for age-associated vascular dysfunction. J Mol Cell Cardiol. 2005. 39:992–995.
11. Lee SK, Chung JI, Park MS, Joo HK, Lee EJ, Cho EJ, Park JB, Ryoo S, Irani K, Jeon BH. Apurinic/apyrimidinic endonuclease 1 inhibits protein kinase C-mediated p66shc phosphorylation and vasoconstriction. Cardiovasc Res. 2011. 91:502–509.
12. Lee SK, Kim HS, Song YJ, Joo HK, Lee JY, Lee KH, Cho EJ, Cho CH, Park JB, Jeon BH. Alteration of p66shc is associated with endothelial dysfunction in the abdominal aortic coarctation of rats. FEBS Lett. 2008. 582:2561–2566.
13. Becker-Hapak M, McAllister SS, Dowdy SF. TAT-mediated protein transduction into mammalian cells. Methods. 2001. 24:247–256.
14. Song YJ, Lee JY, Joo HK, Kim HS, Lee SK, Lee KH, Cho CH, Park JB, Jeon BH. Tat-APE1/ref-1 protein inhibits TNF-alpha-induced endothelial cell activation. Biochem Biophys Res Commun. 2008. 368:68–73.
15. Lee JY, Park KS, Cho EJ, Joo HK, Lee SK, Lee SD, Park JB, Chang SJ, Jeon BH. Human HOXA5 homeodomain enhances protein transduction and its application to vascular inflammation. Biochem Biophys Res Commun. 2011. 410:312–316.
16. Angkeow P, Deshpande SS, Qi B, Liu YX, Park YC, Jeon BH, Ozaki M, Irani K. Redox factor-1: an extra-nuclear role in the regulation of endothelial oxidative stress and apoptosis. Cell Death Differ. 2002. 9:717–725.
17. Wender PA, Mitchell DJ, Pattabiraman K, Pelkey ET, Steinman L, Rothbard JB. The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: peptoid molecular transporters. Proc Natl Acad Sci USA. 2000. 97:13003–13008.
18. Francia P, delli Gatti C, Bachschmid M, Martin-Padura I, Savoia C, Migliaccio E, Pelicci PG, Schiavoni M, Lüscher TF, Volpe M, Cosentino F. Deletion of p66shc gene protects against age-related endothelial dysfunction. Circulation. 2004. 110:2889–2895.
19. Wu Z, Rogers B, Kachi S, Hackett SF, Sick A, Campochiaro PA. Reduction of p66Shc suppresses oxidative damage in retinal pigmented epithelial cells and retina. J Cell Physiol. 2006. 209:996–1005.
20. Anderson TJ. Nitric oxide, atherosclerosis and the clinical relevance of endothelial dysfunction. Heart Fail Rev. 2003. 8:71–86.
21. Kim DD, Sánchez FA, Durán RG, Kanetaka T, Durán WN. Endothelial nitric oxide synthase is a molecular vascular target for the Chinese herb Danshen in hypertension. Am J Physiol Heart Circ Physiol. 2007. 292:H2131–H2137.
22. Cho EJ, Park MS, Kim SS, Kang G, Choi S, Lee YR, Chang SJ, Lee KH, Lee SD, Park JB, Jeon BH. Vasorelaxing activity of ulmus davidiana ethanol extracts in rats: activation of endothelial nitric oxide synthase. Korean J Physiol Pharmacol. 2011. 15:339–344.
23. Shin W, Cuong TD, Lee JH, Min B, Jeon BH, Lim HK, Ryoo S. Arginase inhibition by ethylacetate extract of caesalpinia sappan lignum contributes to activation of endothelial nitric oxide synthase. Korean J Physiol Pharmacol. 2011. 15:123–128.
24. Lee SK, Kim YS, Kim CS, Son SJ, Yoo DG, Lee KH, Lee SD, Park JB, Jeon BH. p66shc adaptor protein suppresses the activation of endothelial nitric oxide synthase in mouse embryonic fibroblasts. Korean J Physiol Pharmacol. 2006. 10:155–159.
25. Grethe S, Ares MP, Andersson T, Pörn-Ares MI. p38 MAPK mediates TNF-induced apoptosis in endothelial cells via phosphorylation and downregulation of Bcl-x(L). Exp Cell Res. 2004. 298:632–642.
26. Yu JH, Kim CS, Yoo DG, Song YJ, Joo HK, Kang G, Jo JY, Park JB, Jeon BH. NADPH oxidase and mitochondrial ROS are involved in the TNF-alpha-induced vascular cell adhesion molecule-1 and monocyte adhesion in cultured endothelial cells. Korean J Physiol Pharmacol. 2006. 10:217–222.
27. Li G, Barrett EJ, Barrett MO, Cao W, Liu Z. Tumor necrosis factor-alpha induces insulin resistance in endothelial cells via a p38 mitogen-activated protein kinase-dependent pathway. Endocrinology. 2007. 148:3356–3363.
28. Zhang XH, Yokoo H, Nishioka H, Fujii H, Matsuda N, Hayashi T, Hattori Y. Beneficial effect of the oligomerized polyphenol oligonol on high glucose-induced changes in eNOS phosphorylation and dephosphorylation in endothelial cells. Br J Pharmacol. 2010. 159:928–938.
29. Xing F, Jiang Y, Liu J, Zhao K, Mo Y, Liu Z, Zeng Y. Downregulation of human endothelial nitric oxide synthase promoter activity by p38 mitogen-activated protein kinase activation. Biochem Cell Biol. 2006. 84:780–788.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download