Abstract
Alcoholic hepatitis is a leading cause of liver failure in which the increased production of tumor necrosis factor α (TNFα) plays a critical role in progression of alcoholic liver disease. In the present study, we investigated the effects of cilostazol, a selective inhibitor of type III phosphodiesterase on ethanol-mediated TNFα production in vitro and in vivo, and the effect of cilostazol was compared with that of pentoxifylline, which is currently used in clinical trial. RAW264.7 murine macrophages were pretreated with ethanol in the presence or absence of cilostazol then, stimulated with lipopolysacchride (LPS). Cilostazol significantly suppressed the level of LPS-stimulated TNFα mRNA and protein with a similar degree to that by pentoxifylline. Cilostazol increased the basal AMP-activated protein kinase (AMPK) activity as well as normalized the decreased AMPK by LPS. AICAR, an AMPK activator and db-cAMP also significantly decreased TNFα production in RAW264.7 cells, but cilostazol did not affect the levels of intracellular cAMP and reactive oxygen species (ROS) production. The in vivo effect of cilostazol was examined using ethanol binge drinking (6 g/kg) mice model. TNFα mRNA and protein decreased in liver from ethanol gavaged mice compared to that from control mice. Pretreatment of mice with cilostazol or pentoxifylline further reduced the TNFα production in liver. These results demonstrated that cilostazol effectively decrease the ethanol-mediated TNFα production both in murine macrophage and in liver from binge drinking mice and AMPK may be responsible for the inhibition of TNFα production by cilostazol.
Alcohol consumption is one of the major causes of liver disease, which is a spectrum including simple alcoholic steatosis, alcoholic hepatitis and cirrhosis. Severe alcoholic hepatitis can be life-threatening and a common reason for hospitalization after heavy alcohol drinking. The progression of alcoholic liver diseases is regulated by various factors and the increased levels of pro-inflammatory factors are known to be important contributors. Heavy alcohol intake causes increase of endotoxin/lipopolysaccharide (LPS) in the blood, which in turn activates Kupffer cells, the resident hepatic macrophage, leading to the increased production of several proinflammatory cytokines, such as TNFα and interleukin-6. In particular, TNFα has been shown to play a critical role in the progression of alcoholic liver disease [1,2]. Previous animal studies have shown that rats treated with TNFα antibody and TNFα receptor I knock-out mice are resistant to alcohol-induced liver injury [3,4]. In addition, the treatment of antibiotics which reduce the level of blood endotoxin prevents the hepatic steatosis and inflammation in rats after exposure to alcohol [5].
A significant amount of studies, therefore, have focused on the regulation of TNFα production in liver exposed to alcohol to develop effective therapies for alcoholic hepatitis. The expression of TNFα in LPS-stimulated Kupffer cell is regulated by a number of signal molecules, including reactive oxygen species (ROS) and cAMP [6,7]. Among the regulators of TNFα expression, it has been clearly demonstrated that the elevation of cyclic AMP (cAMP) suppresses the expression of TNFα in macrophage [8,9]. In consistent with these observations, phosphodiesterase (PDE) inhibitors suppressed TNFα production in macrophage through elevating intracellular cAMP [10-12].
In recent years, pentoxifylline, a non-selective PDE inhibitor has attracted interest in clinical trials and has been pointed as a preferred first-line agent for treatment of alcoholic hepatitis due to its safety compared to glucocorticoids [13]. However, the studies on its efficacy are limited and its effects are unclear in some clinical situations [14]. At this time, no other therapeutic modalities for severe alcoholic hepatitis are available. Therefore, more studies on pentoxifylline are needed as well as new pharmacological therapy is urgent.
Cilostazol is a selective PDE3 inhibitor, which is known to inhibit platelet aggregation by increase of intracellular cAMP. Thus, cilostazol is widely used for treatment of peripheral vascular diseases [15,16]. In addition to its antiplatelet effect, recent studies have suggested its various pharmacologic effects including anti-inflammatory, antioxidant and anti-apoptotic effects via cAMP-dependent and -independent pathways [17-19]. Cilostazol has also shown a beneficial effect on liver steatosis in non-alcoholic fatty liver disease animal model [20].
In the present study, we have examined the effects of cilostazol on ethanol-mediated TNFα expression in in vitro and in vivo model using RAW264.7 murine macrophage and binge drinking mice and the effect of cilostazol was compared to that of pentoxifylline.
Cilostazol was donated by Otsuka Pharmaceuticals (Tokushima, Japan). Dulbecco's modified eagle's medium (DMEM), fetal bovine serum (FBS) and penicillin/streptomycin, and 5-(and-6)-carboxy-2',7'-dichlorodihydrofluorescein diacetate (carboxy-H2DCFDA) were purchased from Invitrogen (Carlsbad, CA, USA). LPS (Escherichia coli 0111:B4), db-cAMP, 5-Aminoimidazole-4-carboxamide 1-β-D-ribofuranoside (AICAR), pentoxifylline, ethanol, carboxymethylcellulose, 3-(4,5-dimethylthiazole-2yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) and protease inhibitors (aprotinin, leupentin, pepstatin A) were obtained from Sigma-Aldrich (St. Louis, MO, USA). The phospho-AMPK antibody was from Cell Signaling Technology (Beverly, MA) and GAPDH antibody was from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
The RAW264.7 murine macrophage was obtained from the Korean Cell Line bank (Seoul, Korea) and cultured in DMEM containing 10% FBS, 100 U/ml penicillin, 100 µg/ml streptomycin and maintained at 37℃ in a humidified incubator with 5% CO2 atmosphere. For experiments, cells were plated at a density of 1×105/cm2 and treated with 25 mM ethanol for 24 h in the presence or absence of cilostazol and pentoxifylline [21,22]. Then, cells were stimulated with 50 ng/ml LPS.
Seven-week old male C57BL/6 (18~22 g) mice were obtained from Central Lab. Animal Inc. (Seoul, Korea). Mice were housed in a specific pathogen-free animal care facility under a 12 h light/dark cycle and were allowed to free access to standard laboratory chow and tab water. Ethanol binge model developed by Carson and Pruett [23] was used. After one week acclimatization, mice were divided into seven groups: control, ethanol (6 g/kg body weight, p.o.), cilostazol (100 mg/kg/day, i.p.), cilostazol (50 and 100 mg/kg/day, i.p.)+ethanol, pentoxifylline (50 and 100 mg/kg/day, i.p.)+ethanol. Mice were administered cilostazol, pentoxifylline, or vehicle (0.5% carboxyl methylcellulose) for 4 days before ethanol administration. Ethanol was diluted with sterile water (32% w/v) and was given orally 1 h after the last treatment with cilostazol or pentoxifylline. Mice were sacrificed 6 h after ethanol administration and liver was collected. The doses of cilostazol or pentoxifylline used in this study were selected by following previous studies reported by others [20,24-26]. The protocol for animal care and use was approved by Animal Care and Use Committee of Yeungnam University.
Cell viability was measured based on the conversion of water soluble tetrazolium MTT to water-insoluble blue formazan by viable cells. Cells were treated with various concentrations of cilostazol or pentoxifylline in the presence or absence of 25 mM ethanol for 24 and 48 h. Then, cells were treated with MTT (5 mg/ml in PBS) and incubated for 4 h. The formation of formazan was dissolved in DMSO and the optical density was measured at 570/620 nm.
Cells were lysed on ice in lysis buffer (20 mM HEPES (pH 7.5), 1 mM EDTA, 1 mM EGTA, 50 mM NaF, 2 mM MgCl2, 150 mM NaCl, 10 mM KCl, 1% NP-40, 1 mM Na3VO4, 1 mM DTT, 1 mM benzamide, 1 mM PMSF, and protease inhibitors). Liver was homogenized in lysis buffer supplemented with 1% glycerol. After centrifugation at 13,000 g, the supernatant was taken and protein concentration was determined by Bradford reagent (Sigma, St. Louis, MO).
The levels of TNFalpha in cell and liver were determined using mouse TNFα ELISA kit (R&D Systems, Inc., Minneapolis, MN). One hundred microliter of cell culture media or liver extract was used and the assay was performed according to protocol provided by manufacturer. The amount of TNFα production was expressed as pg/mg protein.
Total RNA was extracted from RAW264.7 macrophage or liver using Tri reagent (Sigma, St. Louis, MO). RNA was reverse transcribed to cDNA from 1 µg of total RNA using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). For semiquantitative PCR, the following primers were used: TNFα (307 bp: forward, 5'-GGCAGG-TCTACTTTGGAGTCATTGC-3'; reverse, 5'-ACATTCGAGGCTCCAGTGAATTCGG-3'); 18s rRNA (209 bp: forward, 5'-CCCGGGGAGGTAGTGACGAAAAAT-3'; reverse 5'-CGCCCGCTCCCAAGATCCAACTAC-3'). Quantitative real-time PCR was performed using the Real-Time PCR 7500 system and Power SYBR Green PCR master mix (Applied Biosystems) according to the manufacturer's instructions. The thermal cycling conditions were initial incubation at 95℃ for 10 minutes, followed by 45 cycles of 95℃ for 15 seconds, 55℃ for 20 seconds, and 72℃ for 35 seconds. Primers for mouse TNFα (71 bp: forward, 5'-CTATCTCCAGGTTCTCTTCAA-3'; reverse, 5'-GCAGAGAGGAGGTTGACTTTC) and β-actin (121 bp: forward, 5'-TGGACAG-TGAGGCAAGGATAG-3'; reverse, 5'-TACTGCCCTGGCTCCTAGCA-3') were designed using the Primer Express program (Applied Biosystem). The expression level of β-actin was used as an internal control.
To determine ROS generation, FACS analysis was performed. Cells were incubated with 50 µM carboxy-H2DCFDA (Invitrogen, Carlasbad, CA) for 40 min. Cells were washed with PBS and subjected to flow cytometry using a Becton-Dickinson FACS Caliber and analyzed by Cell Quest software (Becton-Dickinson, San Jose, CA).
The concentration of cAMP was measured using cAMP EIA kit (Cayman, Ann Arbor, MI) according to the manufacturer's protocols. Briefly, cells were lysed in 1 ml of lysis buffer and 50 µl of supernatant after centrifugation was used for assay and then the absorbance was measured at 405 nm.
Equal amounts of proteins from cell lysates were separated by 10% SDS-PAGE gel and transferred to nitrocellulose membrane (Bio-Rad, Hercules, CA). The membrane was blocked with 5% non-fat dry milk in Tris-buffered saline. The blots were incubated with primary antibody for phospho-AMPK (Cell signaling, Beverly, MA) and then, reacted with a peroxidase-conjugated secondary antibody. The protein bands were detected using an enhanced chemiluminescence detection system (Millipore, Billerica, MA). The density of respective bands was analyzed by the LAS-3000 imaging system (Fuji film, Tokyo, Japan). The membrane was reprobed with anti-GAPDH antibody, which was used as loading control.
We first examined the cytotoxic effects of cilostazol and pentoxifylline on RAW264.7 cells after ethanol exposure. The treatment of cells with cilostazol (0~100 µM) or pentoxifylline (100 µM) for 24 h did not cause cytotoxicity at any concentration used regardless of the presence of ethanol. At 48 h, pentoxifylline reduced cell viability ~8% and ethanol did not have any effects on cell viability (Fig. 1). In the subsequent experiments, cells were treated with cilostazol or pentixifylline for 24 h.
Next, the effect of cilostazol on LPS-stimulated TNFα expression in the presence of 25 mM ethanol was determined. LPS increased about 260 fold (~29 ng/mg protein) of TNFα production in culture media, which was significantly reduced to 56%, 65% and 76% by pretreatment with 100 µM cilostazol, 50 µM cilostazol and 100 µM pentoxifylline, respectively, compared to DMSO, a vehicle control (Fig. 2A). The level of TNFα mRNA was also substantially reduced by cilostazol and pentoxifylline, showing similar results to that of ELISA (Fig. 2B). These results indicate that cilostazol reduces LPS-stimulated TNFα production in RAW 264.7 cells exposed to ethanol in dose-dependent manner and the degree of its inhibition is comparable to that by pentoxifylline.
Since ROS has been shown to be an important contributor to LPS-stimulated TNFα production in macrophage [7,27], the effect of cilostazol on ROS production in RAW264.7 macrophage was examined to elucidate the underlying mechanisms. The production of ROS was increased about 2 fold by LPS treatment for 4 h (Fig. 3). However, the increase was not ameliorated by pretreatment with cilostazol or pentoxylline. This result indicates that suppression of ROS production is not involved in the action of cilostazol or pentoxifylline. Next, the role of cAMP in the action of cilostazol was examined. As shown in Fig. 4A, pretreatment with db-cAMP, a cell-permeable cAMP analog significantly reduced LPS-stimulated TNFα production by 39%. However, the intracellular cAMP was not changed by cilostazol or pentoxifylline (Fig. 4B), indicating that the inhibition of TNFα by cilostazol occurs via cAMP-independent pathway.
Recent studies have shown the regulation of TNFα expression by AMP-activated protein kinase (AMPK) [28,29]. We therefore examined whether the inhibition of LPS-stimulated TNFα production by cilostazol in macrophage exposed to ethanol was attributable to activation of AMPK. The cells were treated with LPS (50 ng/ml) for different time (0~4 h) and the activation of AMPK was measured by Western blotting. The treatment with LPS decreased AMPK activation about 60% at 30 min (Fig. 4C). In contrast, cilostazol treatment (0~4 h) increased AMPK activation about 2 fold within 5 min and then gradually returned to basal. A pharmacological activator of AMPK, AICAR increased AMPK activation about 30%. The pretreatment with cilostazol increased basal AMPK activity about 2-fold and normalized the level of AMPK activation in LPS-stimulated RAW264.7 cells, whereas pentoxifyllin did not affect the activation of AMPK (Fig. 4D). Consistent with this result, AICAR reduced LPS-stimulated TNFα production in RAW264.7 cells exposed to ethanol (Fig. 4A). These results indicate that AMPK but not cAMP is involved in the inhibition of TNFα production by cilostazol in RAW264.7 cells.
The effects of cilostazol on TNFα production by ethanol was examined in in vivo animal model. Acute ethanol exposure to mice by gavage at a single dose 6 g/kg body weight has shown to significantly increase TNFα production with maximum increase at 6 h after ethanol administration [30,31]. The same protocol was followed in the present study for development of binge drinking model. The levels of TNFα mRNA and protein in liver after ethanol administration were determined by real-time PCR and ELISA, respectively. Unexpectedly, the level of TNFα mRNA was decreased at 6 h after ethanol treatment by 25~30%. The pretreatment with cilostazol (50 and 100 mg/kg) and pentoxifylline (50 and 100 mg/kg) further reduced the TNFα mRNA, although cilostazol itself did not change the level of TNFα expression (Fig. 5A). The ELISA assay also shows similar results (Fig. 5B). These results indicate that cilostazol decreases the expression of TNFα mRNA and protein in liver after ethanol exposure in vivo with similar extent to pentoxifylline, although ethanol treatment reduced the level of TNFα in this model.
The results from this study have shown that cilostazol significantly suppressed LPS-stimulated TNFα production in RAW 264.7 murine macrophage exposed to ethanol and in liver from binge drinking mice.
Numerous clinical and experimental studies have shown that the increased level of hepatic TNFα and monocyte TNFα production in alcoholic hepatitis are positively correlated with disease severity [2,32]. Chronic ethanol exposure enhances LPS-stimulated TNF production in RAW264.7 cells as well as Kupffer cells [21,22]. In accordance with this observation, inhibition of TNFα by TNFα antibody reduced liver injury in alcohol fed rats [3]. Recent clinical reports have provided beneficial effects of pentoxifylline, a TNFα suppressor for treatment of alcoholic hepatitis and have suggested this treatment as an alternative to glucocorticoids [13]. At this time, however, its efficacy on survival benefit is controversial [14,33]. It is necessary to conduct more studies on the effects of pentoxifylline while the development of new pharmacological therapy for alcoholic hepatitis is needed. Our results show that cilostazol significantly suppresses LPS-stimulated TNFα production in ethanol-primed RAW264.7 as well as binge drinking mice and the extent of inhibition by cilostazol is comparable to that of pentoxifylline. The overproduction of TNFα by ethanol has been shown to be associated with increased TNF mRNA expression [22,34]. In agreement with these results, the level of TNFα mRNA was increased by ethanol and LPS in RAW264.7 cells, which was decreased by cilostazol and pentoxifylline. These data suggest that both cilostazol and pentoxifylline suppress the production of TNFalpha at transcription level.
The underlying mechanisms by which ethanol increases TNFα expression in macrophage have been extensively studied. LPS increases ROS production in Kupffer cells and ethanol exposure further increase LPS-induced ROS production, which leads to increase of TNFα production [7]. We have also observed that LPS simulation increases ROS production in RAW264.7 cells exposed to ethanol. However, neither cilostazol nor pentoxifylline reduced LPS-stimulated ROS production. This indicates that the inhibition of TNFα production by cilostazol occurs via ROS-independent pathway. Although previous study has shown that cilostazol attenuates NADPH oxidase-derived ROS production in macrophage [35], intracellular ROS can be generated from various sources including NADPH oxidase and mitochondrial electron transport chain [36]. A lot of studies have shown that mitochondria plays a critical role in ethanol-induced ROS production and liver injury [37,38]. Furthermore, Chandel et al. [27], have shown that antioxidants had no effect on LPS-induced TNFα expression in murine macrophage.
Enhanced intracellular cAMP has been well known to suppress LPS-stimulated TNFα production in macrophage in vivo and in vitro [39,40]. Moreover, chronic ethanol exposure results in decrease of cAMP, which is one of mechanisms responsible for increased TNFα production from Kupffer cells and macrophages [21,22]. Concomitant with these observations, previous studies have documented that various cAMP elevating agents including adenylyl cyclase activator and PDE inhibitors invariably suppress TNFα production in macrophages. Our data also shows that db-cAMP significantly reduced TNFα production in RAW264.7 cells, supporting previous reports by others. However, cilostazol and pentoxifylline did not increase intracellular cAMP levels in the present study, indicating that cilostazol inhibition of TNFα production in ethanol treated macrophage is cAMP-independent. Similarly, previous studies have also shown that cilostazol did not change intracellular cAMP in RAW264.7 macrophage [19,24].
The activation of AMPK has been shown to repress inflammatory responses stimulated with LPS in vitro and in vivo [28,29]. Recent studies have shown that the anti-inflammatory effects of cilostazol in vascular smooth muscle cells and endothelial cells occur by activation of AMPK pathway which is independent of cAMP [28,29]. Our data support this findings demonstrating that AICAR, an activator of AMPK suppressed ethanol-mediated TNFα production and cilostazol enhanced LPS decreased AMPK activation in RAW264.7 cells exposed to ethanol. These results suggest that cilostazol exerts the inhibitory effects on TNFα production in ethanol-primed RAW264.7 cells through activation of AMPK.
Various alcohol binge animal models have been employed to study alcoholic hepatitis [41,42]. In preliminary experiments, we observed significant increase in serum ALT/AST, makers of liver injury and hepatic TNFα mRNA expression 6 h after gavage of a single dose of 6 g/kg or three doses of 5 g/kg ethanol, similar results to previous reports by others [31,42]. In subsequent repeated experiments, however, we obtained the opposite results that TNFα expression was rather decreased in mice gavaged with 6 g/kg ethanol compared with that in control mice. Recent study has shown similar finding that the administration of either a single dose or three dose of ethanol (5 g/kg) to rats suppressed TNFα expression in liver collected 4 h after ethanol gavage [41]. Several previous studies have demonstrated that binge ethanol causes dual effects, pro- and anti-inflammatory responses; mostly anti-inflammatory effects at early after ethanol treatment (0~6 hr) and pro-inflammatory effects later after ethanol treatment (after 24 hr) [43,44]. In addition, the differential effects of binge alcohol drinking on hepatic TNFα production in animal model may be due to different animal species and different animal batches used in experiments. Although we failed to present the increase of hepatic TNFα expression by ethanol binge itself, our data clearly show that cilostazol does decrease the expression of TNFα in liver exposed to ethanol in vivo and the degree of inhibition by cilostazol is similar to that by pentoxifylline. However, additional studies to examine the effect of cilostazol on the TNFα production in ethanol-fed animal model are needed to make concrete conclusion.
Taken together, these data demonstrate that cilostazol suppresses ethanol-mediated TNFα expression in RAW264.7 macrophage and in liver from binge drinking mice and the effects of cilostazol involves AMPK activation. Furthermore, the effects of cilostazol are comparable to that of pentoxifylline.
Figures and Tables
Fig. 1
The effects of cilostazol and pentoxifylline on viability of RAW264.7 cells. Cells were pretreated with cilostazol (0~100 µM) or pentoxifylline (100 µM) for 1 h followed by treatment with 25 mM ethanol or vehicle control (culture media) for 24 h (A) and 48 h (B). Data represented as percentage of cell survival over the control cells are mean±SEM of four independent experiments. *p<0.05 vs. control (CTZ, cilostazol; PTX, pentoxifylline; EtOH, ethanol).

Fig. 2
The effects of cilostazol and pentoxifylline on LPS-stimulated TNFα expression in RAW264.7 cells exposed to ethanol. Cells were treated with 25 mM ethanol in presence of cilostazol (50 and 100 µM), pentoxifylline (100 µM) or DMSO (vehicle control) for 24 h. Then, cells were stimulated with 50 ng/ml LPS for 4 h. (A) The accumulation of TNFα in cell culture media was measured by ELISA and normalized by the amount of protein of each sample. Data represented as percentage of TNFα production over DMSO group are mean±SEM of four independent experiments. (B) The level of TNFα mRNA was measured by RT-PCR. *p<0.05 vs. DMSO group (CTZ, cilostazol; PTX, pentoxifylline).
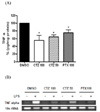
Fig. 3
The effects of cilostazol and pentoxifylline on LPS-induced ROS production in RAW264.7 cells exposed to ethanol. Cells were treated with 25 mM ethanol in presence of cilostazol (100 µM), pentoxifylline (100 µM) or DMSO for 24 h. Then, cells were stimulated with 50 ng/ml LPS for 4 h. After incubation of cells with 50 µM carboxy-H2DCFDA for 40 min, the production of ROS was measured by flow cytometry. Data represented as percentage increases over the DMSO control and are expressed as mean±SEM of three independent experiments. *p<0.05, **p<0.01 vs. control. #p<0.05 vs. corresponding DMSO-treated cells (Con, control; CTZ, cilostazol; PTX, pentoxifylline).
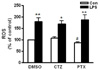
Fig. 4
The role of cAMP and AMPK in the effects of cilostazol on inhibition of TNFα production in RAW264.7 cells. (A) Cells were treated with 25 mM ethanol in presence of cilostazol (100 µM), db-cAMP (100 µM), AICAR (1 mM) or DMSO for 24 h and then, stimulated with 50 ng/ml LPS for 4 h. The level of TNFα production from cell culture media was measured by ELISA and normalized by the amount of protein of each sample. Data are represented as percentage of TNFα production over LPS-stimulated cells in vehicle control (DMSO) group and are expressed as mean±SEM of three independent experiments. (B) Cells were treated with cilostazol, pentoxifylline and db-cAMP for 15 min, and then intracellular cAMP was measured. The concentrations of cAMP (pmol/ml) are represented as fold increase over the control and are expressed as mean±SEM of three independent experiments. (C) Cells were treated with 50 ng/ml LPS or 100 µM cilostazol for different time (0~4 h) and 1 mM AICAR for 1 h, or (D) cells were treated with 50 ng/ml LPS in the presence or absence of cilostazol (100 µM) or pentoxifylline (100 µM) for 30 min. Whole cell extracts were prepared and the activation of AMPK was detected by Western blotting. The blots are representative of three independent experiments. *p<0.05, **p<0.01 vs. DMSO control group (CTZ, cilostazol; PTX, pentoxifylline).

Fig. 5
The effects of cilostazol and pentoxifylline on TNFα expression in liver from binge drinking mice. Mice were treated with cilostazol (50 and 100 mg/kg) or pentoxifyline (50 and 100 mg/kg) for 4 days by intraperitoneal injection and control mice were treated with vehicle (0.5% carboxyl methylcellulose). Then, mice were intragastrically administered with 6 g/kg ethanol and sacrificed 6 h after ethanol administration. Liver was collected to measure TNFα mRNA (A) and protein (B) by real-time PCR and ELISA, respectively. Data are represented as mean±SEM (n=5~6 mice). *p<0.05 vs. control group (CTZ, cilostazol; PTX, pentoxifylline; EtOH, ethanol).

ACKNOWLEDGEMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (No. 2009-0069506).
References
1. Thurman RG. II. Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am J Physiol. 1998. 275:G605–G611.
2. Vidali M, Stewart SF, Albano E. Interplay between oxidative stress and immunity in the progression of alcohol-mediated liver injury. Trends Mol Med. 2008. 14:63–71.
3. Iimuro Y, Gallucci RM, Luster MI, Kono H, Thurman RG. Antibodies to tumor necrosis factor alfa attenuate hepatic necrosis and inflammation caused by chronic exposure to ethanol in the rat. Hepatology. 1997. 26:1530–1537.
4. Yin M, Wheeler MD, Kono H, Bradford BU, Gallucci RM, Luster MI, Thurman RG. Essential role of tumor necrosis factor alpha in alcohol-induced liver injury in mice. Gastroenterology. 1999. 117:942–952.
5. Adachi Y, Bradford BU, Gao W, Bojes HK, Thurman RG. Inactivation of Kupffer cells prevents early alcohol-induced liver injury. Hepatology. 1994. 20:453–460.
6. Tannenbaum CS, Hamilton TA. Lipopolysaccharide-induced gene expression in murine peritoneal macrophages is selectively suppressed by agents that elevate intracellular cAMP. J Immunol. 1989. 142:1274–1280.
7. Thakur V, Pritchard MT, McMullen MR, Wang Q, Nagy LE. Chronic ethanol feeding increases activation of NADPH oxidase by lipopolysaccharide in rat Kupffer cells: role of increased reactive oxygen in LPS-stimulated ERK1/2 activation and TNF-alpha production. J Leukoc Biol. 2006. 79:1348–1356.
8. Fischer W, Schudt C, Wendel A. Protection by phosphodiesterase inhibitors against endotoxin-induced liver injury in galactosamine-sensitized mice. Biochem Pharmacol. 1993. 45:2399–2404.
9. Beavo JA, Brunton LL. Cyclic nucleotide research-still expanding after half a century. Nat Rev Mol Cell Biol. 2002. 3:710–718.
10. Smith KR, Leonard D, McDonald JD, Tesfaigzi Y. Inflammation, mucous cell metaplasia, and Bcl-2 expression in response to inhaled lipopolysaccharide aerosol and effect of rolipram. Toxicol Appl Pharmacol. 2011. 253:253–260.
11. Michel O, Dentener M, Cataldo D, Cantinieaux B, Vertongen F, Delvaux C, Murdoch RD. Evaluation of oral corticosteroids and phosphodiesterase-4 inhibitor on the acute inflammation induced by inhaled lipopolysaccharide in human. Pulm Pharmacol Ther. 2007. 20:676–683.
12. Yoshikawa M, Suzumura A, Tamaru T, Takayanagi T, Sawada M. Effects of phosphodiesterase inhibitors on cytokine production by microglia. Mult Scler. 1999. 5:126–133.
13. Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000. 119:1637–1648.
14. Louvet A, Diaz E, Dharancy S, Coevoet H, Texier F, Thévenot T, Deltenre P, Canva V, Plane C, Mathurin P. Early switch to pentoxifylline in patients with severe alcoholic hepatitis is inefficient in non-responders to corticosteroids. J Hepatol. 2008. 48:465–470.
15. Kambayashi J, Liu Y, Sun B, Shakur Y, Yoshitake M, Czerwiec F. Cilostazol as a unique antithrombotic agent. Curr Pharm Des. 2003. 9:2289–2302.
16. Liu Y, Shakur Y, Kambayashi J. Phosphodiesterases as targets for intermittent claudication. Handb Exp Pharmacol. 2011. 204:211–236.
17. Lee JH, Oh GT, Park SY, Choi JH, Park JG, Kim CD, Lee WS, Rhim BY, Shin YW, Hong KW. Cilostazol reduces atherosclerosis by inhibition of superoxide and tumor necrosis factor-alpha formation in low-density lipoprotein receptor-null mice fed high cholesterol. J Pharmacol Exp Ther. 2005. 313:502–509.
18. Kim MJ, Lee JH, Park SY, Hong KW, Kim CD, Kim KY, Lee WS. Protection from apoptotic cell death by cilostazol, phosphodiesterase type III inhibitor, via cAMP-dependent protein kinase activation. Pharmacol Res. 2006. 54:261–267.
19. Park WS, Jung WK, Lee DY, Moon C, Yea SS, Park SG, Seo SK, Park C, Choi YH, Kim GY, Choi JS, Choi IW. Cilostazol protects mice against endotoxin shock and attenuates LPS-induced cytokine expression in RAW 264.7 macrophages via MAPK inhibition and NF-kappaB inactivation: not involved in cAMP mechanisms. Int Immunopharmacol. 2010. 10:1077–1085.
20. Fujita K, Nozaki Y, Wada K, Yoneda M, Endo H, Takahashi H, Iwasaki T, Inamori M, Abe Y, Kobayashi N, Kirikoshi H, Kubota K, Saito S, Nagashima Y, Nakajima A. Effectiveness of antiplatelet drugs against experimental non-alcoholic fatty liver disease. Gut. 2008. 57:1583–1591.
21. Shi L, Kishore R, McMullen MR, Nagy LE. Chronic ethanol increases lipopolysaccharide-stimulated Egr-1 expression in RAW 264.7 macrophages: contribution to enhanced tumor necrosis factor alpha production. J Biol Chem. 2002. 277:14777–14785.
22. Gobejishvili L, Barve S, Joshi-Barve S, Uriarte S, Song Z, McClain C. Chronic ethanol-mediated decrease in cAMP primes macrophages to enhanced LPS-inducible NF-kappaB activity and TNF expression: relevance to alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2006. 291:G681–G688.
23. Carson EJ, Pruett SB. Development and characterization of a binge drinking model in mice for evaluation of the immunological effects of ethanol. Alcohol Clin Exp Res. 1996. 20:132–138.
24. Nakaya K, Ayaori M, Uto-Kondo H, Hisada T, Ogura M, Yakushiji E, Takiguchi S, Terao Y, Ozasa H, Sasaki M, Komatsu T, Ohsuzu F, Ikewaki K. Cilostazol enhances macrophage reverse cholesterol transport in vitro and in vivo. Atherosclerosis. 2010. 213:135–141.
25. Robin MA, Demeilliers C, Sutton A, Paradis V, Maisonneuve C, Dubois S, Poirel O, Lettéron P, Pessayre D, Fromenty B. Alcohol increases tumor necrosis factor alpha and decreases nuclear factor-kappab to activate hepatic apoptosis in genetically obese mice. Hepatology. 2005. 42:1280–1290.
26. Kudo H, Takahara T, Yata Y, Kawai K, Zhang W, Sugiyama T. Lipopolysaccharide triggered TNF-alpha-induced hepatocyte apoptosis in a murine non-alcoholic steatohepatitis model. J Hepatol. 2009. 51:168–175.
27. Chandel NS, Trzyna WC, McClintock DS, Schumacker PT. Role of oxidants in NF-kappa B activation and TNF-alpha gene transcription induced by hypoxia and endotoxin. J Immunol. 2000. 165:1013–1021.
28. Aoki C, Hattori Y, Tomizawa A, Jojima T, Kasai K. Anti-inflammatory role of cilostazol in vascular smooth muscle cells in vitro and in vivo. J Atheroscler Thromb. 2010. 17:503–509.
29. Hattori Y, Suzuki K, Tomizawa A, Hirama N, Okayasu T, Hattori S, Satoh H, Akimoto K, Kasai K. Cilostazol inhibits cytokine-induced nuclear factor-kappaB activation via AMP-activated protein kinase activation in vascular endothelial cells. Cardiovasc Res. 2009. 81:133–139.
30. Lambert JC, Zhou Z, Wang L, Song Z, McClain CJ, Kang YJ. Preservation of intestinal structural integrity by zinc is independent of metallothionein in alcohol-intoxicated mice. Am J Pathol. 2004. 164:1959–1966.
31. Zhou Z, Wang L, Song Z, Lambert JC, McClain CJ, Kang YJ. A critical involvement of oxidative stress in acute alcohol-induced hepatic TNF-alpha production. Am J Pathol. 2003. 163:1137–1146.
32. Sougioultzis S, Dalakas E, Hayes PC, Plevris JN. Alcoholic hepatitis: from pathogenesis to treatment. Curr Med Res Opin. 2005. 21:1337–1346.
33. Mathurin P. Corticosteroids for alcoholic hepatitis--what's next? J Hepatol. 2005. 43:526–533.
34. Hill DB, Barve S, Joshi-Barve S, McClain C. Increased monocyte nuclear factor-kappaB activation and tumor necrosis factor production in alcoholic hepatitis. J Lab Clin Med. 2000. 135:387–395.
35. Yun MR, Park HM, Seo KW, Kim CE, Yoon JW, Kim CD. Cilostazol attenuates 4-hydroxynonenal-enhanced CD36 expression on murine macrophages via inhibition of NADPH oxidase-derived reactive oxygen species production. Korean J Physiol Pharmacol. 2009. 13:99–106.
36. Li Y, Trush MA. Diphenyleneiodonium, an NAD(P)H oxidase inhibitor, also potently inhibits mitochondrial reactive oxygen species production. Biochem Biophys Res Commun. 1998. 253:295–299.
37. Hoek JB, Pastorino JG. Ethanol, oxidative stress, and cytokine-induced liver cell injury. Alcohol. 2002. 27:63–68.
38. Chacko BK, Srivastava A, Johnson MS, Benavides GA, Chang MJ, Ye Y, Jhala N, Murphy MP, Kalyanaraman B, Darley-Usmar VM. Mitochondria-targeted ubiquinone (MitoQ) decreases ethanol-dependent micro and macro hepatosteatosis. Hepatology. 2011. 54:153–163.
39. Zidek Z. Adenosine - cyclic AMP pathways and cytokine expression. Eur Cytokine Netw. 1999. 10:319–328.
40. Haraguchi S, Good RA, Day-Good NK. A potent immunosuppressive retroviral peptide: cytokine patterns and signaling pathways. Immunol Res. 2008. 41:46–55.
41. Aroor AR, Jackson DE, Shukla SD. Elevated activation of ERK1 and ERK2 accompany enhanced liver injury following alcohol binge in chronically ethanol-fed rats. Alcohol Clin Exp Res. 2011. 35:2128–2138.
42. Song Z, Deaciuc I, Song M, Lee DY, Liu Y, Ji X, McClain C. Silymarin protects against acute ethanol-induced hepatotoxicity in mice. Alcohol Clin Exp Res. 2006. 30:407–413.
43. Yamashina S, Wheeler MD, Rusyn I, Ikejima K, Sato N, Thurman RG. Tolerance and sensitization to endotoxin in Kupffer cells caused by acute ethanol involve interleukin-1 receptor-associated kinase. Biochem Biophys Res Commun. 2000. 277:686–690.
44. Nishiyama D, Ikejima K, Honda H, Hirose M, Takei Y, Sato N. Acute ethanol administration down-regulates toll-like receptor-4 in the murine liver. Hepatol Res. 2002. 23:130–137.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download