Abstract
Ginsenosides are low molecular weight glycosides found in ginseng that exhibit neuroprotective effects through inhibition of N-methyl-D-aspartic acid (NMDA) receptor channel activity. Ginsenosides, like other natural compounds, are metabolized by gastric juices and intestinal microorganisms to produce ginsenoside metabolites. However, little is known about how ginsenoside metabolites regulate NMDA receptor channel activity. In the present study, we investigated the effects of ginsenoside metabolites, such as compound K (CK), protopanaxadiol (PPD), and protopanaxatriol (PPT), on oocytes that heterologously express the rat NMDA receptor. NMDA receptor-mediated ion current (INMDA) was measured using the 2-electrode voltage clamp technique. In oocytes injected with cRNAs encoding NMDA receptor subunits, PPT, but not CK or PPD, reversibly inhibited INMDA in a concentration-dependent manner. The IC50 for PPT on INMDA was 48.1±4.6 µM, was non-competitive with NMDA, and was independent of the membrane holding potential. These results demonstrate the possibility that PPT interacts with the NMDA receptor, although not at the NMDA binding site, and that the inhibitory effects of PPT on INMDA could be related to ginseng-mediated neuroprotection.
Glutamate is a major excitatory neurotransmitter in the central nervous system (CNS) [1]. Glutamate not only interacts with GTP-binding protein coupled receptors (i.e., metabotropic glutamate receptor subtypes), but also binds to various ionotropic ligand-gated glutamate receptors, which include α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), kainite, and N-methyl-D-aspartic acid (NMDA) receptors [1,2]. NMDA receptors play a pivotal role in the regulation of synaptic function in the CNS [3]. In the hippocampus, NMDA receptors have important roles in physiological functions such as learning and memory. In abnormal conditions, overstimulation of NMDA receptors causes nervous system excitotoxicity [4,5], NMDA receptor inhibitors are currently under investigation for clinical applications.
Ginseng, the root of Panax ginseng C.A. Meyer, has been used as a general tonic to promote longevity and enhance body functions to protect against stress, fatigue, diseases, cancer, and diabetes mellitus [6]. Recent studies have shown that, among the various ginseng components, ginsenosides (also called ginseng saponins) exhibit neuroprotective effects [7]. For example, we demonstrated that ginsenoside Rg3 attenuated NMDA receptor-mediated ion currents as well as NMDA-mediated excitotoxicity in cultured neurons [8,9]. In addition, we also demonstrated that Rg3 inhibited voltagegated Ca2+ and Na+ channel currents, which are also activated following NMDA receptor activation [10-13]. However, orally administered ginsenosides can be broken down by intestinal microorganisms [14]. Protopanaxadiol (PPD) ginsenosides are metabolized by intestinal microorganisms into compound K (CK), which has 1 glucose at the C-20 position, and are further metabolized to form PPD, which has no glucose. In contrast, protopanaxatriol (PPT) ginsenosides are metabolized to PPT, which only has a ginsenoside backbone and no carbohydrate components (Fig. 1). A recent report showed that these ginsenoside metabolites might also induce apoptosis of cancer cells and function as an anti-cancer agent [15], suggesting that ginsenosides are the prodrugs of these metabolites. However, relatively little is known about how ginsenoside metabolites regulate receptor channel activity.
The purpose of this study was to investigate how ginsenoside metabolites affect NMDA receptor-mediated ion current (INMDA). We report here that PPT inhibited INMDA, while CK and PPD had no effect on INMDA.
The ginsenoside metabolites CK, PPD, and PPT were purchased from AMBO Institute (Seoul, Korea) (Fig. 1A). All other reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA).
X. laevis frogs were purchased from Xenopus I (Ann Arbor, MI, USA). Animal care and handling were carried out in accordance with the highest standards of the Konkuk University guidelines. To isolate oocytes, frogs were anesthetized with an aerated solution of 3-amino benzoic acid ethyl ester, and the ovarian follicles were removed. The oocytes were separated by treatment with collagenase followed by agitation for 2 h in Ca2+-free OR2 medium containing 82.5 mM NaCl, 2 mM KCl, 1 mM MgCl2, 5 mM HEPES, 2.5 mM sodium pyruvate, 100 units/ml penicillin, and 100 µg/ml streptomycin. Stage V~VI oocytes were collected and stored in ND96 medium (96 mM NaCl, 2 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, and 5 mM HEPES, pH 7.5) supplemented with 50 µg/ml gentamicin. The oocyte-containing solution was maintained at 18℃ with continuous gentle shaking and was replaced daily. Electrophysiological experiments were performed 3 to 5 days after oocyte isolation. For the NMDA receptor experiments, NMDA receptor subunit-encoding cRNAs (40 nl) were injected into the animal or vegetal pole of each oocyte 1 day after isolation using a 10-µl microdispenser (VWR Scientific, West Chester, PA, USA) fitted with a tapered glass pipette tip (15~20 µm in diameter) [16].
A custom-made Plexiglas net chamber was used for 2-electrode voltage-clamp recordings, as previously reported [16]. A single oocyte was constantly superfused during recording with a recording solution (96 mM NaCl, 2 mM KCl, 0.3 mM CaCl2, and 5 mM HEPES, pH 7.6) in the presence or absence of NMDA or ginsenoside metabolites. The microelectrodes were filled with 3 M KCl and had a resistance of 0.2~0.7 MΩ. Two-electrode voltage-clamp recordings were obtained at room temperature using an oocyte clamp (OC-725C; Warner Instrument, Hamden, CT, USA), and were digitized using Digidata 1200A (Molecular Devices, Sunnyvale, CA, USA). Stimulation and data acquisition were controlled using pClamp 8 software (Molecular Devices). For most electrophysiological data, the oocytes were clamped at a holding potential of -60 mV. To determine the current and voltage (I-V) relationship, voltage ramps were applied from -100 to +50 mV for 1 s. In the different membrane-holding potential experiments, the oocytes were clamped at the indicated holding potentials. Linear leak and capacitance currents were corrected by the leak subtraction procedure.
To obtain the concentration-response curve for the effect of PPT on the inward peak INMDA mediated by the NMDA receptor, the INMDA peak was plotted at different concentrations of PPT. Origin software version 8.0 (OriginLab Corp., Northampton, MA, USA) was used to fit the plot to the Hill equation: I/Imax=1/[1+(IC50/[A])nH], where Imax was maximal current obtained from the ED50 value of the NMDA receptor, IC50 was the concentration of PPT required to decrease the response by 50%, [A] was the concentration of PPT, and nH was the Hill coefficient. All values were presented as the mean±SEM. The differences between the of the control and treatment data were determined using the paired Student's t-test. A p-value of less than 0.05 was considered statistically significant.
In the present study, we examined the effect of the ginsenoside metabolites CK, PPD, and PPT on NMDA receptor channel activity. We expressed the rat NMDA receptor NR1b and NR2B subunits in Xenopus oocytes [17]. As shown in Fig. 1B, the addition of NMDA to the bathing solution induced a large inward current (INMDA) in oocytes injected with rat NMDA receptor subunit cRNAs. In H2O-injected control oocytes, the application of NMDA did not induce any inward currents (data not shown). CK, PPD, or PPT alone also had no effect in oocytes expressing the NMDA receptor at a holding potential of -60 mV (data not shown). However, co-application of PPT, but not CK or PPD, (at 100 µM) with NMDA (300 µM; with 10 µM glycine, a co-agonist required for efficient opening of the ion channel [18]) for 40 s inhibited INMDA in oocytes expressing NMDA receptor in a reversible manner (Fig. 2, n=6~9 from 3 different frogs). In concentration-dependent experiments, co-application of PPT with NMDA for 40 s decreased INMDA in a concentration-dependent manner in oocytes expressing the NMDA receptor (Fig. 2). Therefore, co-application of PPT at 10, 30, 100, 300, and 1,000 µM decreased INMDA in oocytes expressing the NMDA receptor by 6.5±11.0%, 20.3±10.3%, 35.8±6.2%, 47.6±7.3%, and 50.8±8.9%, respectively. The IC50 for PPT on INMDA was 48.1±4.6 µM (Fig. 2, n= 10~11, with samples taken from 3 different frogs for each point).
To examine the current-voltage relationship, the membrane potential was held at -60 mV, and a voltage ramp was applied from -100 to +50 mV for 1 s. In the absence of NMDA, the inward current at -100 mV was less than 0.2 µA, and the outward current at +50 mV was approximately 0.2 µA (Fig. 3). The addition of NMDA to the glycine containing bathing medium induced a mainly inward current at negative voltages and an outward current at positive voltages [19]. The reversal potentials were near -10 mV for responses up to 300 µM NMDA (with 10 µM glycine) both in the presence and absence of PPT (50 µM). Although co-application of PPT and NMDA decreased the currents, it did not affect the NMDA receptor channel property because PPT did not change the reversal potential of the NMDA receptor (Fig. 3). To further examine the mechanism by which co-application of PPT inhibits INMDA in oocytes expressing the NMDA receptor, we analyzed the effect of PPT on INMDA evoked by different concentrations of NMDA (at a fixed glycine concentration of 10 µM) (Fig. 4A). Co-application of PPT (50 µM) for 40 s with different concentrations of NMDA significantly shifted the concentration-response curve of NMDA to the right (EC50 values changed from 109.7±2.5 µM to 172.0±3.3 µM, p<0.05). However, the Hill coefficient did not change significantly (1.27±0.27 to 1.22±0.17), indicating that the number of PPT molecules interacting with NMDA receptor did not change. Therefore, PPT-mediated inhibition of INMDA was not affected by increasing concentrations of NMDA ranging from 30 to 300 µM (Fig. 4A), suggesting that PPT-mediated inhibition of INMDA might be non-competitive.
Since PPT-mediated inhibition of INMDA is not competitive with NMDA, we next examined whether PPT-mediated inhibition of INMDA is dependent on the membrane holding potential.
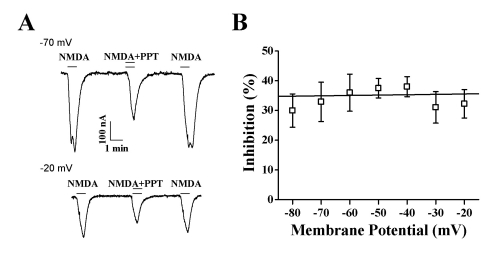
At different holding potentials, the INMDA elicited by 300 µM NMDA (with 10 µM glycine) and 50 µM PPT was measured. Our results revealed that PPT-mediated inhibition of INMDA in oocytes expressing the rat NMDA receptor was independent of the membrane holding potential (Fig. 5B). At membrane holding potentials of -80, -70, -60, -50, -40, -30, and -20 mV, PPT inhibited INMDA by 29.9±5.6%, 32.9±6.6%, 35.9±6.2%, 37.4±3.2%, 38.0±3.3%, 31.0±5.3%, and 32.2±4.8%, respectively (n=9~12, from 3 different frogs), indicating that PPT-induced regulations of NMDA receptor channel activity is not achieved through interaction with channel pore region (Fig. 5B).
Ginsenosides consist of aglycone and carbohydrates portions. The aglycone is the backbone of the ginsenoside, consisting of a hydrophobic 4-ring steroid-like structure that can be non-polar, and carbohydrates on carbons-3, 6, and 20 of the backbone that are polar (Fig. 1A). In vitro and in vivo studies have shown that orally administered ginsenosides are metabolized and ultimately become aglycones such as PPD and PPT [14,15].
We investigated the effects of ginsenoside metabolites on the rat NMDA receptor heterologously expressed in Xenopus oocytes. We found that: (1) co-application of PPT but not CK or PPD along with NMDA reversibly inhibited INMDA in a concentration-dependent manner; (2) PPT-mediated INMDA inhibition was independent of the concentration of NMDA. In other words, a high concentration of NMDA did not attenuate PPT action. Finally, (3) PPT-mediated inhibition of INMDA was membrane holding potential-independent, since the effect of PPT on INMDA was not affected by more depolarizing membrane potentials. These results suggested that PPT might not interact with the NMDA binding site or the channel pore region when exerting its effect on INMDA.
In previous reports, we showed that ginsenoside metabolites such as CK, PPD, and PPT differentially regulate ion channels and receptors. For example, CK but not PPT strongly inhibited the voltage-dependent α1G-type Ca2+ channel [10]. Similarly, we found that CK, but not PPT, inhibited a neuronal Na+ (Nav1.2) channel [12,13]. Recently, we also reported that although PPD itself had no effects on human HERG K+ channel activity, it inhibited the decelerating effects on HERG K+ channel currents mediated by ginsenoside Rg3 [20]. In contrast, with ligand-gated ion channels, we demonstrated that PPT, but not CK, inhibited 5-HT3A receptor-mediated currents [21], and that both CK and PPT inhibited α3β4 nicotinic acetylcholine receptor-mediated currents [22]. In the present study, we found that PPT inhibited NMDA receptor-mediated ion currents. Although the previous and present findings demonstrate that ginsenosides and ginsenoside metabolites have regulatory effects on voltage-dependent and ligand-gated ion channel activities, they have differential effects on the regulation of ion channels and receptors.
Interestingly, the previous reports showed that ginsenoside Rg3, a protopanaxadiol ginsenoside, inhibited NMDA receptor-mediated ion currents in cultured hippocampal neurons [8,9]. Rg3 is metabolized to PPD, a backbone ginsenoside structure without carbohydrates. However, in the present study, PPD had no effect on INMDA. Therefore, the carbohydrate portion attached to carbon-3 of Rg3 might play a key role in Rg3-mediated NMDA receptor regulation (Fig. 1). In contrast, protopanaxatriol ginsenosides, which have carbohydrates at carbon-6 and/or -20, induce minimal inhibition of NMDA receptor-mediated ion currents in neurons [8]. However, PPT, a metabolite of protopanaxatriol ginsenosides, strongly inhibited INMDA (Fig. 1B). The chemical structure of PPD and PPT differ slightly; PPT has hydroxyl group at carbon-6, but PPD does not (Fig. 1A). Therefore, the hydroxyl group on carbon-6 of PPT might play a key role in PPT-mediated INMDA inhibition (Fig. 1). However, elucidation of the exact mechanisms underlying the regulation of NMDA receptor by ginsenoside Rg3 or ginsenoside metabolites will require further studies of the structure-function relationship of PPT and Rg3.
Overstimulation of NMDA receptors in the nervous system is closely related to neurotoxicity, neurodegenerative diseases, and ischemia [23-25]. Ginsenosides attenuate these excitotoxin-mediated insults both in vitro and in vivo [7]. The inhibitory effects of a ginsenoside metabolite, PPT, on NMDA receptor channel activity might be one of the main contributors to neuroprotection against NMDA receptor-related neurotoxicity after ginsenoside intake and anti-cancer activity [8,9]. However, it has been reported that the majority of ginsenosides are rapidly metabolized in gastric juice, the intestine, and the liver, and most ginsenoside metabolites are excreted in the urine [26,27]. The plasma concentration of ginsenoside metabolites produced after oral administration of ginseng or ginsenosides might be low, including PPT, which exhibits various in vitro neuropharmacological actions as shown in the present study. Therefore, we need to carefully explain the beneficial effects of ginsenoside metabolites such as PPT in the nervous system.
In conclusion, when we examined the effects of ginsenoside metabolites on the INMDA of the rat NMDA receptor expressed in Xenopus oocytes, we found that PPT inhibited INMDA in a concentration-dependent, non-competitive, and membrane holding potential-independent manner. Our results indicate that PPT, a metabolite of protopanaxatriol ginsenosides, could be a novel agent interacting with the NMDA receptor, and that the inhibition of INMDA by PPT could provide a molecular basis for the pharmacological actions of ginsenoside metabolites in the nervous system.
References
1. Moriyoshi K, Masu M, Ishii T, Shigemoto R, Mizuno N, Nakanishi S. Molecular cloning and characterization of the rat NMDA receptor. Nature. 1991; 354:31–37. PMID: 1834949.

2. Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994; 17:31–108. PMID: 8210177.

3. Carroll RC, Zukin RS. NMDA-receptor trafficking and targeting: implications for synaptic transmission and plasticity. Trends Neurosci. 2002; 25:571–577. PMID: 12392932.

5. Soundarapandian MM, Tu WH, Peng PL, Zervos AS, Lu Y. AMPA receptor subunit GluR2 gates injurious signals in ischemic stroke. Mol Neurobiol. 2005; 32:145–155. PMID: 16215279.

6. Nah SY. Ginseng: recent advances and trends. Korean J Ginseng Sci. 1997; 21:1–12.
7. Nah SY, Kim DH, Rhim H. Ginsenosides: are any of them candidates for drugs acting on the central nervous system? CNS Drug Rev. 2007; 13:381–404. PMID: 18078425.

8. Kim S, Ahn K, Oh TH, Nah SY, Rhim H. Inhibitory effect of ginsenosides on NMDA receptor-mediated signals in rat hippocampal neurons. Biochem Biophys Res Commun. 2002; 296:247–254. PMID: 12163009.

9. Kim S, Kim T, Ahn K, Park WK, Nah SY, Rhim H. Ginsenoside Rg3 antagonizes NMDA receptors through a glycine modulatory site in rat cultured hippocampal neurons. Biochem Biophys Res Commun. 2004; 323:416–424. PMID: 15369768.
10. Lee JH, Jeong SM, Kim JH, Lee BH, Yoon IS, Lee JH, Choi SH, Lee SM, Park YS, Lee JH, Kim SS, Kim HC, Lee BY, Nah SY. Effects of ginsenosides and their metabolites on voltage-dependent Ca2+ channel subtypes. Mol Cells. 2006; 21:52–62. PMID: 16511347.
11. Choi SH, Lee JH, Pyo MK, Lee BH, Shin TJ, Hwang SH, Kim BR, Lee SM, Oh JW, Kim HC, Bae CS, Rhim H, Nah SY. Mutations Leu427, Asn428, and Leu431 residues within transmembrane domain-I-segment 6 attenuate ginsenoside-mediated L-type Ca2+ channel current inhibitions. Biol Pharm Bull. 2009; 32:1224–1230. PMID: 19571390.
12. Lee JH, Jeong SM, Kim JH, Lee BH, Yoon IS, Lee JH, Choi SH, Kim DH, Rhim H, Kim SS, Kim JI, Jang CG, Song JH, Nah SY. Characteristics of ginsenoside Rg3-mediated brain Na+ current inhibition. Mol Pharmacol. 2005; 68:1114–1126. PMID: 16014805.
13. Lee JH, Lee BH, Choi SH, Yoon IS, Shin TJ, Pyo MK, Lee SM, Kim HC, Nah SY. Involvement of batrachotoxin binding sites in ginsenoside-mediated voltage-gated Na+ channel regulation. Brain Res. 2008; 1203:61–67. PMID: 18321475.
14. Hasegawa H, Suzuki R, Nagaoka T, Tezuka Y, Kadota S, Saiki I. Prevention of growth and metastasis of murine melanoma through enhanced natural-killer cytotoxicity by fatty acid-conjugate of protopanaxatriol. Biol Pharm Bull. 2002; 25:861–866. PMID: 12132658.

15. Wakabayashi C, Murakami K, Hasegawa H, Murata J, Saiki I. An intestinal bacterial metabolite of ginseng protopanaxadiol saponins has the ability to induce apoptosis in tumor cells. Biochem Biophys Res Commun. 1998; 246:725–730. PMID: 9618279.

16. Lee BH, Jeong SM, Lee JH, Kim JH, Yoon IS, Lee JH, Choi SH, Lee SM, Chang CG, Kim HC, Han Y, Paik HD, Kim Y, Nah SY. Quercetin inhibits the 5-hydroxytryptamine type 3 receptor-mediated ion current by interacting with pre-transmembrane domain I. Mol Cells. 2005; 20:69–73. PMID: 16258243.
17. Zheng X, Zhang L, Wang AP, Bennett MV, Zukin RS. Ca2+ influx amplifies protein kinase C potentiation of recombinant NMDA receptors. J Neurosci. 1997; 17:8676–8686. PMID: 9348336.
18. Durand GM, Bennett MV, Zukin RS. Splice variants of the N-methyl-D-aspartate receptor NR1 identify domains involved in regulation by polyamines and protein kinase C. Proc Natl Acad Sci USA. 1993; 90:6731–6735. PMID: 8341692.

19. Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seeburg PH. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992; 256:1217–1221. PMID: 1350383.

20. Choi SH, Shin TJ, Hwang SH, Lee BH, Kang J, Kim HJ, Oh JW, Bae CH, Lee SH, Nah SY. Differential effects of ginsenoside metabolites on HERG K+ channel currents. J Ginseng Res. 2011; 35:191–199.
21. Lee BH, Jeong SM, Lee JH, Kim DH, Kim JH, Kim JI, Shin HC, Lee SM, Nah SY. Differential effect of ginsenoside metabolites on the 5-HT3A receptor-mediated ion current in Xenopus oocytes. Mol Cells. 2004; 17:51–56. PMID: 15055527.
22. Lee JH, Jeong SM, Lee BH, Kim DH, Kim JH, Kim JI, Lee SM, Nah SY. Differential effect of bovine serum albumin on ginsenoside metabolite-induced inhibition of alpha3beta4 nicotinic acetylcholine receptor expressed in Xenopus oocytes. Arch Pharm Res. 2003; 26:868–873. PMID: 14609137.
24. Ikonomidou C, Turski L. Neurodegenerative disorders: clues from glutamate and energy metabolism. Crit Rev Neurobiol. 1996; 10:239–263. PMID: 8971131.

25. Lee HG, Zhu X, Ghanbari HA, Ogawa O, Raina AK, O'Neill MJ, Perry G, Smith MA. Differential regulation of glutamate receptors in Alzheimer's disease. Neurosignals. 2002; 11:282–292. PMID: 12566929.

26. Qian T, Cai Z, Wong RN, Mak NK, Jiang ZH. In vivo rat metabolism and pharmacokinetic studies of ginsenoside Rg3. J Chromatogr B Analyt Technol Biomed Life Sci. 2005; 816:223–232.

27. Zhao Q, Zheng X, Jiang J, Zhou H, Hu P. Determination of ginsenoside Rg3 in human plasma and urine by high performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2010; 878:2266–2273.
Fig. 1
Chemical Structure of Ginsenoside Rg3 and Ginsenoside Metabolites and the Effects of Ginsenoside Metabolites on INMDA. (A) Chemical structure of ginsenoside Rg3 and ginsenoside metabolites. (B) Effect of ginsenoside metabolites on INMDA in oocytes expressing the rat NMDA receptor. Application of NMDA (300 µM) and glycine (10 µM) elicited INMDA. Co-application of PPT (100 µM) but not CK (100 µM) or PPD (100 µM) with NMDA (300 µM) and glycine (10 µM) attenuated INMDA. The traces represent 6 separate oocytes.
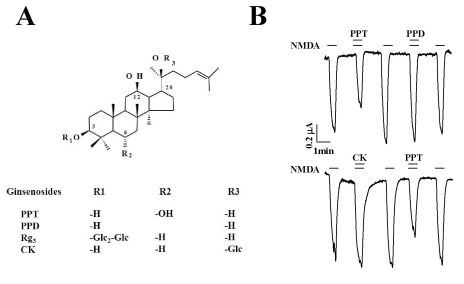
Fig. 2
Concentration-dependent Effects of PPT Co-application with NMDA (300 µM) and Glycine (10 µM) on INMDA. PPT inhibited INMDA in a concentration-dependent manner. The traces represent 6 separate oocytes.
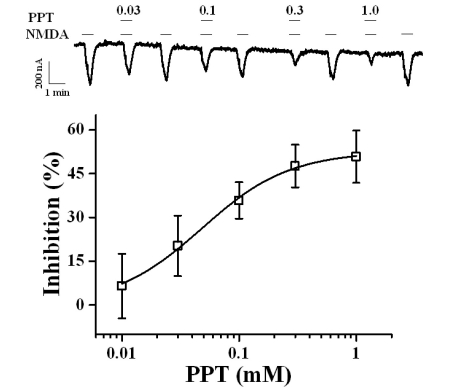
Fig. 3
Current-voltage Relationship of PPT-mediated INMDA Inhibition of the NMDA Receptor. Representative current-voltage relationships were obtained using voltage ramps of -100 to +50 mV for 1 s at a holding potential of -60 mV. Voltage steps were applied before and after application of 300 µM NMDA and 10 µM glycine in the presence or absence of 50 µM PPT. The reversal potential for the receptor was -14.70±0.9 mV and -13.82±0.9 mV in the presence and absence of PPT, respectively.
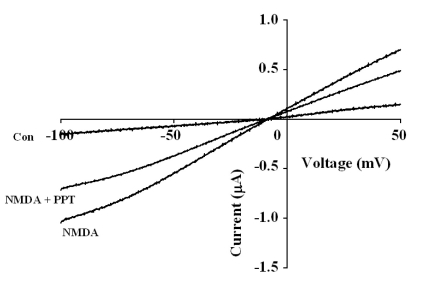
Fig. 4
Concentration-dependent Effects of NMDA on PPT-mediated Inhibition of INMDA. (A) The representative traces shown for the indicated concentrations of NMDA represent 6 separate oocytes from 3 different batches of frogs. (B) Concentration-response relationships for NMDA in NMDA receptors treated with NMDA (10~1000 µM; with 10 µM glycine) or with NMDA plus 50 µM PPT in oocytes expressing the rat NMDA receptor. The normalized INMDA of oocytes expressing the NMDA receptor was measured at the indicated concentrations of NMDA in the presence (○) or absence (□) of 50 µM PPT. Oocytes were exposed to NMDA alone or NMDA and PPT for 1 min prior to application. Oocytes were voltage-clamped at a holding potential of -60 mV.
Fig. 5
Voltage-independent PPT-mediated INMDA Inhibition of the NMDA Receptor. The effect of PPT (50 µM) on the response to NMDA (300 µM, with 10 µM glycine) was determined in oocytes expressing NR1b/NR2A. (A) Representative traces showing inhibition by 50 µM PPT. (B) Summary of percent inhibition induced by PPT at the indicated membrane holding potentials in oocytes expressing the NMDA receptor. Each point represents the mean±SEM. (n=6~8/group).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download