Abstract
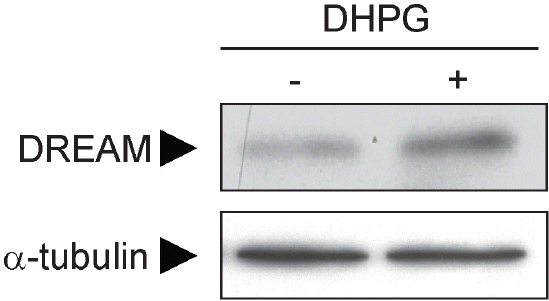
DREAM (downstream regulatory element antagonistic modulator) is a calcium-binding protein that regulates dynorphin expression, promotes potassium channel surface expression, and enhances presenilin processing in an expression level-dependent manner. However, no molecular mechanism has yet explained how protein levels of DREAM are regulated. Here we identified group I mGluR (mGluR1/5) as a positive regulator of DREAM protein expression. Overexpression of mGluR1/5 increased the cellular level of DREAM. Up-regulation of DREAM resulted in increased DREAM protein in both the nucleus and cytoplasm, where the protein acts as a transcriptional repressor and a modulator of its interacting proteins, respectively. DHPG (3,5-dihydroxyphenylglycine), a group I mGluR agonist, also up-regulated DREAM expression in cortical neurons. These results suggest that group I mGluR is the first identified receptor that may regulate DREAM activity in neurons.
References
1. Buxbaum JD. A role for calsenilin and related proteins in multiple aspects of neuronal function. Biochem Biophys Res Commun. 2004; 322:1140–1144.

2. Cheng HY, Pitcher GM, Laviolette SR, Whishaw IQ, Tong KI, Kockeritz LK, Wada T, Joza NA, Crackower M, Goncalves J, Sarosi I, Woodgett JR, Oliveira-dos-Santos AJ, Ikura M, van der Kooy D, Salter MW, Penninger JM. DREAM is a critical transcriptional repressor for pain modulation. Cell. 2002; 108:31–43.

3. Buxbaum JD, Choi EK, Luo Y, Lilliehook C, Crowley AC, Merriam DE, Wasco W. Calsenilin: a calcium-binding protein that interacts with the presenilins and regulates the levels of a presenilin fragment. Nat Med. 1998; 4:1177–1181.

4. An WF, Bowlby MR, Betty M, Cao J, Ling HP, Mendoza G, Hinson JW, Mattsson KI, Strassle BW, Trimmer JS, Rhodes KJ. Modulation of A-type potassium channels by a family of calcium sensors. Nature. 2000; 403:553–556.

5. Carrión AM, Link WA, Ledo F, Mellström B, Naranjo JR. DREAM is a Ca2+-regulated transcriptional repressor. Nature. 1999; 398:80–84.
6. Jo DG, Lee JY, Hong YM, Song S, Mook-Jung I, Koh JY, Jung YK. Induction of pro-apoptotic calsenilin/DREAM/KChIP3 in Alzheimer's disease and cultured neurons after amyloid-beta exposure. J Neurochem. 2004; 88:604–611.
7. Zaidi NF, Thomson EE, Choi EK, Buxbaum JD, Wasco W. Intracellular calcium modulates the nuclear translocation of calsenilin. J Neurochem. 2004; 89:593–601.

8. 8. Woo HN, Chang JW, Choi YH, Gwon AR, Jung YK, Jo DG. Characterization of subcellular localization and Ca2+ modulation of calsenilin/DREAM/KChIP3. Neuroreport. 2008; 19:1193–1197.
9. Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010; 50:295–322.

10. Ribeiro FM, Paquet M, Cregan SP, Ferguson SS. Group I metabotropic glutamate receptor signalling and its implication in neurological disease. CNS Neurol Disord Drug Targets. 2010; 9:574–595.
11. Dölen G, Carpenter RL, Ocain TD, Bear MF. Mechanism-based approaches to treating fragile X. Pharmacol Ther. 2010; 127:78–93.

12. Cho CH, Shin HK. Spinal metabotropic glutamate receptors (mGluRs) are involved in the melittin-induced nociception in rats. Korean J Physiol Pharmacol. 2008; 12:237–243.

13. Krystal JH, Mathew SJ, D'Souza DC, Garakani A, Gunduz-Bruce H, Charney DS. Potential psychiatric applications of metabotropic glutamate receptor agonists and antagonists. CNS Drugs. 2010; 24:669–693.

14. Brackmann M, Zhao C, Kuhl D, Manahan-Vaughan D, Braunewell KH. MGluRs regulate the expression of neuronal calcium sensor proteins NCS-1 and VILIP-1 and the immediate early gene arg3.1/arc in the hippocampus in vivo. Biochem Biophys Res Commun. 2004; 322:1073–1079.
15. Osawa M, Dace A, Tong KI, Valiveti A, Ikura M, Ames JB. Mg2+ and Ca2+ differentially regulate DNA binding and dimerization of DREAM. J Biol Chem. 2005; 280:18008–18014.
16. Francesconi A, Duvoisin RM. Role of the second and third intracellular loops of metabotropic glutamate receptors in mediating dual signal transduction activation. J Biol Chem. 1998; 273:5615–5624.

17. Lee JH, Lee J, Choi KY, Hepp R, Lee JY, Lim MK, Chatani-Hinze M, Roche PA, Kim DG, Ahn YS, Kim CH, Roche KW. Calmodulin dynamically regulates the trafficking of the metabotropic glutamate receptor mGluR5. Proc Natl Acad Sci USA. 2008; 105:12575–12580.

18. Burgoyne RD. Neuronal calcium sensor proteins: generating diversity in neuronal Ca2+ signalling. Nat Rev Neurosci. 2007; 8:182–193.
19. McGarrigle D, Huang XY. GPCRs signaling directly through Src-family kinases. Sci STKE. 2007; 2007::p. e35.

20. Sun Y, McGarrigle D, Huang XY. When a G protein-coupled receptor does not couple to a G protein. Mol Biosyst. 2007; 3:849–854.

21. Costigan M, Woolf CJ. No DREAM, No pain. Closing the spinal gate. Cell. 2002; 108:297–300.
22. Kolber BJ, Montana MC, Carrasquillo Y, Xu J, Heinemann SF, Muglia LJ, Gereau RW 4th. Activation of metabotropic glutamate receptor 5 in the amygdala modulates pain-like behavior. J Neurosci. 2010; 30:8203–8213.

23. Walker K, Bowes M, Panesar M, Davis A, Gentry C, Kesingland A, Gasparini F, Spooren W, Stoehr N, Pagano A, Flor PJ, Vranesic I, Lingenhoehl K, Johnson EC, Varney M, Urban L, Kuhn R. Metabotropic glutamate receptor subtype 5 (mGlu5) and nociceptive function. I. Selective blockade of mGlu5 receptors in models of acute, persistent and chronic pain. Neuropharmacology. 2001; 40:1–9.
24. Sevostianova N, Danysz W. Analgesic effects of mGlu1 and mGlu5 receptor antagonists in the rat formalin test. Neuropharmacology. 2006; 51:623–630.

25. Hu HJ, Alter BJ, Carrasquillo Y, Qiu CS, Gereau RW 4th. Metabotropic glutamate receptor 5 modulates nociceptive plasticity via extracellular signal-regulated kinase-Kv4.2 signaling in spinal cord dorsal horn neurons. J Neurosci. 2007; 27:13181–13191.
Fig. 1.
Group I mGluR increases DREAM protein expression. (A, C, and D∼F) HEK293 cells were co-transfected with 500 ng of each plasmid expressing DREAM or hippocalcin and 50 ng of pRK5 empty vector, pRK5-mGluR subtypes, or pRK5-mGluR5 F767S (Gαq binding site mutant of mGluR5). Cell lysates were immunoblotted with rabbit anti-DREAM or anti-hippocalcin antibody. (B) pRK5 or pRK5-mGluR5 (200 ng) was transfected into SK-N-MC cells for 36 h before cell lysis. Immunoblotting using mouse anti-DREAM antibody was performed. (E) Cells were treated with MPEP for 15 h before lysis. Lysis was performed by boiling with denaturing lysis buffer after 36 h of cultivation. WT, wild-type.
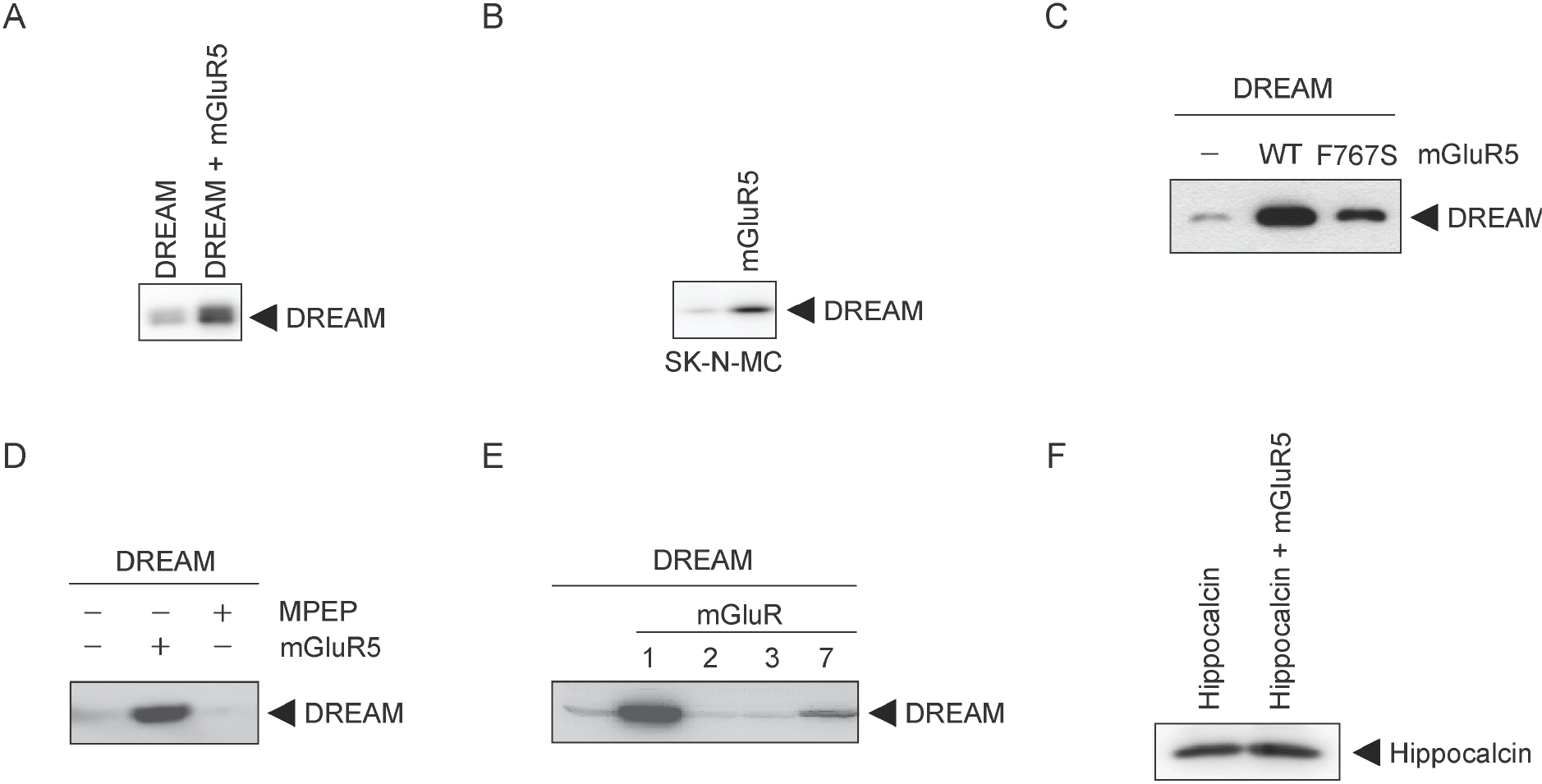
Fig. 2.
mGluR5 stabilizes DREAM protein. (A) pCBA-DREAM was co-transfected with pRK5 or pRK5-mGluR5 into HEK293 cells. Cells were lysed after 48 h of incubation. Cells were treated with cycloheximide (CHX) at 100 μg/ml for 0, 2, 4, 6, or 8 h before cell lysis. DREAM expression was analyzed by immunoblotting with rabbit anti-DREAM antibody. (B) HEK293 cells were transfected as indicated above. EDTA-AM (100 nM) was added to culture for 12 h to chelate intracellular Ca2+. Immunoblotting was performed using rabbit anti-DREAM antibody. (C) Lysates from cells expressing mGluR5 and DREAM (E186Q/E234Q) double mutant or DREAM (D150N/E186Q/E234Q) triple mutant were subjected to Western blotting.
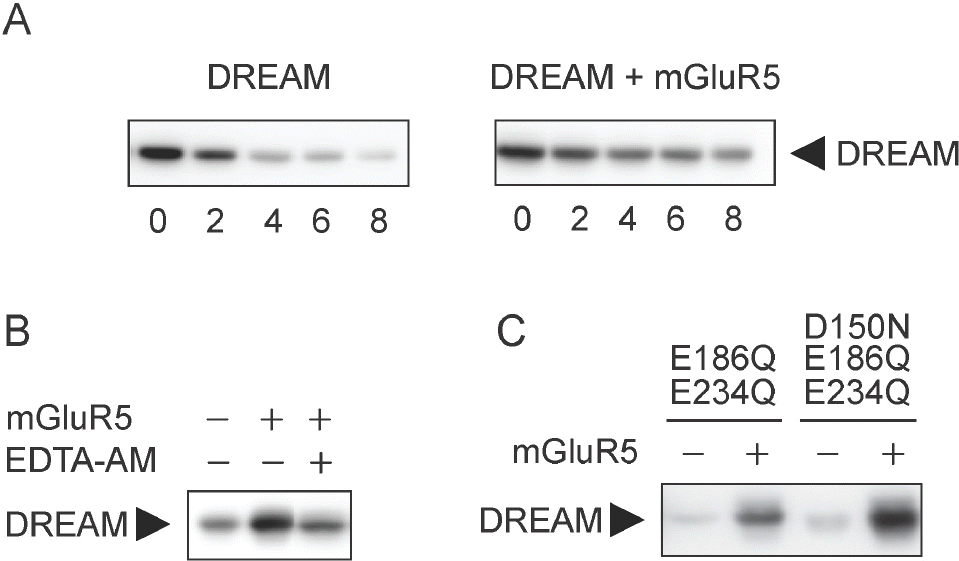
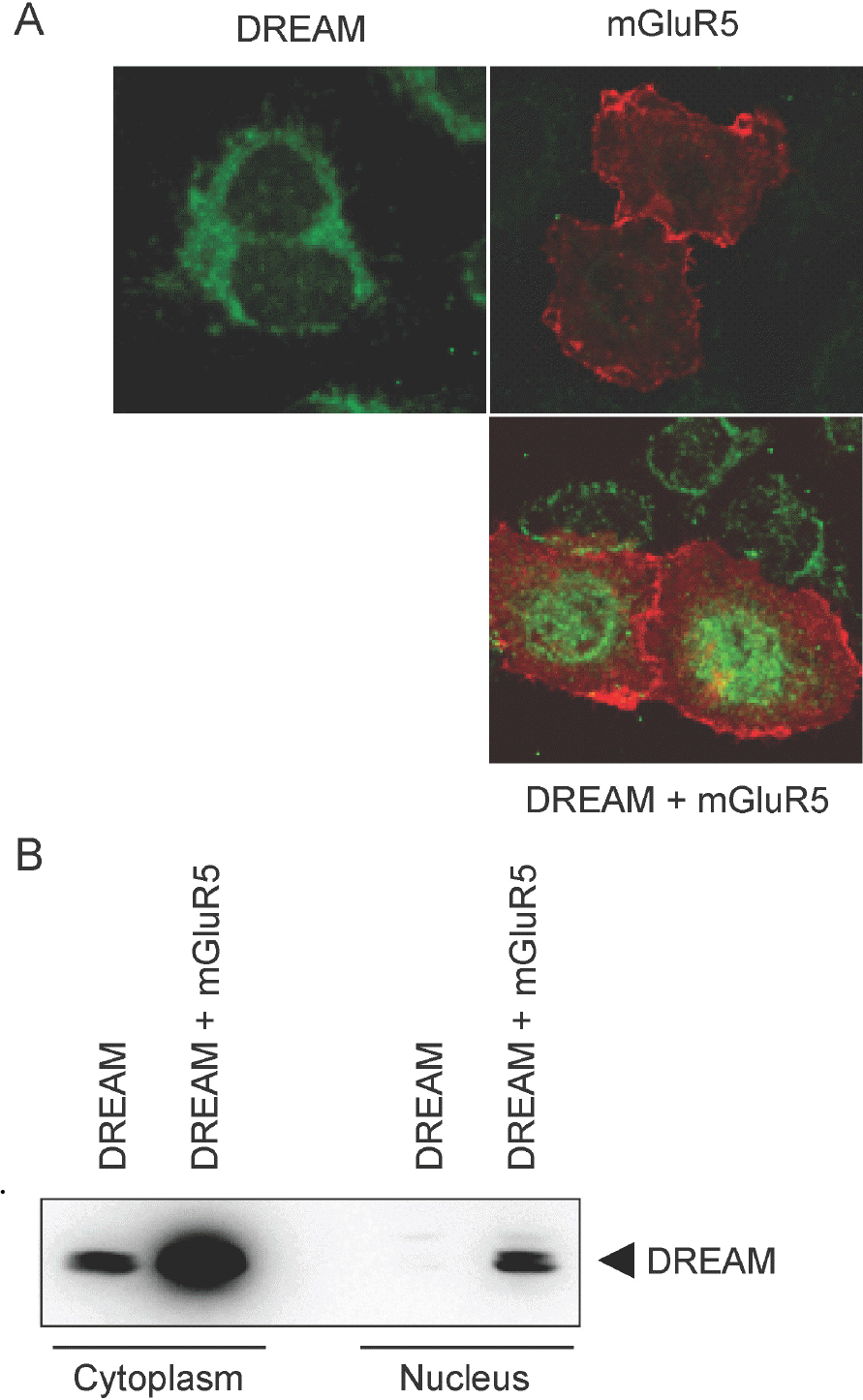
Fig. 3.
Subcellular localization of increased DREAM by mGluR5. (A) pCBA-DREAM and/or pRK5-myc-mGluR5 were transfected into HeLa cells. Following 36 h incubation, cells were fixed with 4% paraformaldehyde, blocked with goat serum, and incubated with rabbit anti-DREAM and anti-myc antibody. After incubation, fixed cells were washed and treated again with Alexa 488 anti-rabbit and Alexa 568 anti-mouse antibodies. Images were obtained under a Zeiss LSM 710 confocal microscope. (B) HEK293 cells were cotransfected with pRK5-mGluR5 and pCBA-DREAM. Cytoplasmic and nuclear extracts were prepared from transfected cells. Western blotting was performed to determine the amount of DREAM in each fraction.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download