Abstract
P2Y receptors are metabotropic G-protein-coupled receptors, which are involved in many important biologic functions in the central nervous system including retina. Subtypes of P2Y receptors in retinal tissue vary according to the species and the cell types. We examined the molecular and pharmacologic profiles of P2Y purinoceptors in retinoblastoma cell, which has not been identified yet. To achieve this goal, we used Ca2+ imaging technique and western blot analysis in WERI-Rb-1 cell, a human retinoblastoma cell line. ATP (10 μM) elicited strong but transient [Ca2+]i increase in a concentration-dependent manner from more than 80% of the WERI-Rb-1 cells (n=46). Orders of potency of P2Y agonists in evoking [Ca2+]i transients were 2MeS-ATP> ATP>> UTP=αβ-MeATP, which was compatible with the subclass of P2Y1 receptor. The [Ca2+]i transients evoked by applications of 2MeS-ATP and/or ATP were also profoundly suppressed in the presence of P2Y1 selective blocker (MRS 2179; 30 μM). P2Y1 receptor expression in WERI-Rb-1 cells was also identified by using western blot. Taken together, P2Y1 receptor is mainly expressed in a retinoblastoma cell, which elicits Ca2+ release from internal Ca2+ storage sites via the phospholipase C-mediated pathway. P2Y1 receptor activation in retinoblastoma cell could be a useful model to investigate the role of purinergic [Ca2+]i signaling in neural tissue as well as to find a novel therapeutic target to this lethal cancer.
References
1. Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998; 50:413–492.
3. Hwang SM, Koo NY, Choi SY, Chun GS, Kim JS, Park K. P2X7 Receptor-mediated membrane blebbing in salivary epithelial cells. Korean J Physiol Pharmacol. 2009; 13:175–179.

4. von Kügelgen I, Wetter A. Molecular pharmacology of P2Y-receptors. Naunyn Schmiedebergs Arch Pharmacol. 2000; 362:310–323.

5. Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006; 58:281–341.

6. Boeynaems JM, Communi D, Savi P, Herbert JM. P2Y receptors: in the middle of the road. Trends Pharmacol Sci. 2000; 21:1–3.

7. Janssens R, Boeynaems JM. Effects of extracellular nucleotides and nucleosides on prostate carcinoma cells. Br J Pharmacol. 2001; 132:536–546.

8. Fries JE, Wheeler-Schilling TH, Kohler K, Guenther E. Distribution of metabotropic P2Y receptors in the rat retina: a single-cell RT-PCR study. Brain Res Mol Brain Res. 2004; 130:1–6.

9. Cowlen MS, Zhang VZ, Warnock L, Moyer CF, Peterson WM, Yerxa BR. Localization of ocular P2Y2 receptor gene expression by in situ hybridization. Exp Eye Res. 2003; 77:77–84.

10. Fries JE, Goczalik IM, Wheeler-Schilling TH, Kohler K, Guenther E, Wolf S, Wiedemann P, Bringmann A, Reichenbach A, Francke M, Pannicke T. Identification of P2Y receptor subtypes in human muller glial cells by physiology, single cell RT-PCR, and immunohistochemistry. Invest Ophthalmol Vis Sci. 2005; 46:3000–3007.

11. Fries JE, Wheeler-Schilling TH, Guenther E, Kohler K. Expression of P2Y1, P2Y2, P2Y4, and P2Y6 receptor subtypes in the rat retina. Invest Ophthalmol Vis Sci. 2004; 45:3410–3417.

12. Li Y, Holtzclaw LA, Russell JT. Müller cell Ca2+ waves evoked by purinergic receptor agonists in slices of rat retina. J Neurophysiol. 2001; 85:986–994.
13. Sanches G, de Alencar LS, Ventura AL. ATP induces proliferation of retinal cells in culture via activation of PKC and extracellular signal-regulated kinase cascade. Int J Dev Neurosci. 2002; 20:21–27.

14. Ward MM, Fletcher EL. Subsets of retinal neurons and glia express P2Y1 receptors. Neuroscience. 2009; 160:555–566.

15. Pereira Tde O, da Costa GN, Santiago AR, Ambrósio AF, dos Santos PF. High glucose enhances intracellular Ca2+ responses triggered by purinergic stimulation in retinal neurons and microglia. Brain Res. 2010; 1316:129–138.
16. Sholl-Franco A, Fragel-Madeira L, Macama Ada C, Linden R, Ventura AL. ATP controls cell cycle and induces proliferation in the mouse developing retina. Int J Dev Neurosci. 2010; 28:63–73.
17. McFall RC, Nagy RM, Nagle BT, McGreevy LM. Scanning electron microscopic observation of two retinoblastoma cell lines. Cancer Res. 1978; 38:2827–2835.
18. Dyer MA, Bremner R. The search for the retinoblastoma cell of origin. Nat Rev Cancer. 2005; 5:91–101.

19. Barnes S, Haynes LW. Low-voltage-activated calcium channels in human retinoblastoma cells. Brain Res. 1992; 598:19–22.

20. Xia SL, Wang L, Cash MN, Teng X, Schwalbe RA, Wingo CS. Extracellular ATP-induced calcium signaling in mIMCD-3 cells requires both P2X and P2Y purinoceptors. Am J Physiol Renal Physiol. 2004; 287:F204–214.

21. Ciapa B, Pesando D, Wilding M, Whitaker M. Cell-cycle calcium transients driven by cyclic changes in inositol trisphosphate levels. Nature. 1994; 368:875–878.

22. Berridge MJ. Calcium signal transduction and cellular control mechanisms. Biochim Biophys Acta. 2004; 1742:3–7.

23. Burnstock G. Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol Rev. 2006; 58:58–86.

24. Guo Y, Hong YJ, Jang HJ, Kim MJ, Rhie DJ, Jo YH, Hahn SJ, Yoon SH. Octyl gallate inhibits ATP-induced intracellular calcium increase in PC12 cells by inhibiting multiple pathways. Korean J Physiol Pharmacol. 2010; 14:21–28.

25. Kim DR, Rah SH, Sohn JH, Yeh BI, Ko CM, Park JS, Kim MJ, Lee JW, Kong ID. Calcium mobilization by activation of M(3)/M(5) muscarinic receptors in the human retinoblastoma. J Pharmacol Sci. 2007; 105:184–192.

26. Wheeler-Schilling TH, Marquordt K, Kohler K, Guenther E, Jabs R. Identification of purinergic receptors in retinal ganglion cells. Brain Res Mol Brain Res. 2001; 92:177–180.

27. Peterson WM, Meggyesy C, Yu K, Miller SS. Extracellular ATP activates calcium signaling, ion, and fluid transport in retinal pigment epithelium. J Neurosci. 1997; 17:2324–2337.

28. Sullivan DM, Erb L, Anglade E, Weisman GA, Turner JT, Csaky KG. Identification and characterization of P2Y2 nucleotide receptors in human retinal pigment epithelial cells. J Neurosci Res. 1997; 49:43–52.

29. Ryan JS, Baldridge WH, Kelly ME. Purinergic regulation of cation conductances and intracellular Ca2+ in cultured rat retinal pigment epithelial cells. J Physiol. 1999; 520:745–759.
30. Kimura K, Nishimura T, Satoh Y. Effects of ATP and its analogues on [Ca2+]i dynamics in the rabbit corneal epithelium. Arch Histol Cytol. 1999; 62:129–138.
31. Cha SH, Hahn TW, Sekine T, Lee KH, Endou H. Purinoceptor-mediated calcium mobilization and cellular proliferation in cultured bovine corneal endothelial cells. Jpn J Pharmacol. 2000; 82:181–187.

32. Shiue MH, Kulkarni AA, Gukasyan HJ, Swisher JB, Kim KJ, Lee VH. Pharmacological modulation of fluid secretion in the pigmented rabbit conjunctiva. Life Sci. 2000; 66::p. L105–111.

33. Collison DJ, Duncan G. Regional differences in functional receptor distribution and calcium mobilization in the intact human lens. Invest Ophthalmol Vis Sci. 2001; 42:2355–2363.
34. Shahidullah M, Wilson WS. Mobilisation of intracellular calcium by P2Y2 receptors in cultured, non-transformed bovine ciliary epithelial cells. Curr Eye Res. 1997; 16:1006–1016.

36. Murakami T, Fujihara T, Nakamura M, Nakata K. P2Y(2) receptor stimulation increases tear fluid secretion in rabbits. Curr Eye Res. 2000; 21:782–787.

37. Mundasad MV, Novack GD, Allgood VE, Evans RM, Gorden JC, Yerxa BR. Ocular safety of INS365 ophthalmic solution: a P2Y(2) agonist in healthy subjects. J Ocul Pharmacol Ther. 2001; 17:173–179.

38. Meyer CH, Hotta K, Peterson WM, Toth CA, Jaffe GJ. Effect of INS37217, a P2Y(2) receptor agonist, on experimental retinal detachment and electroretinogram in adult rabbits. Invest Ophthalmol Vis Sci. 2002; 43:3567–3574.
39. Fujihara T, Murakami T, Nagano T, Nakamura M, Nakata K. INS365 suppresses loss of corneal epithelial integrity by secretion of mucin-like glycoprotein in a rabbit short-term dry eye model. J Ocul Pharmacol Ther. 2002; 18:363–370.

40. Nour M, Quiambao AB, Peterson WM, Al-Ubaidi MR, Naash MI. P2Y(2) receptor agonist INS37217 enhances functional recovery after detachment caused by subretinal injection in normal and rds mice. Invest Ophthalmol Vis Sci. 2003; 44:4505–4514.
Fig. 1.
ATP-evoked Ca2+ signaling in WERI-Rb-1 cells. (A) Original trace of Ca2+ responses evoked by four different concentrations of ATP in the same group of cells. (B) Dose-response curve fitting the peak responses obtained from five different groups of cells. F0: basal fluorescence after Fluo 3-AM loading, F: change in fluorescence after introducing ATP, n=total cell number, N=number of experiment.
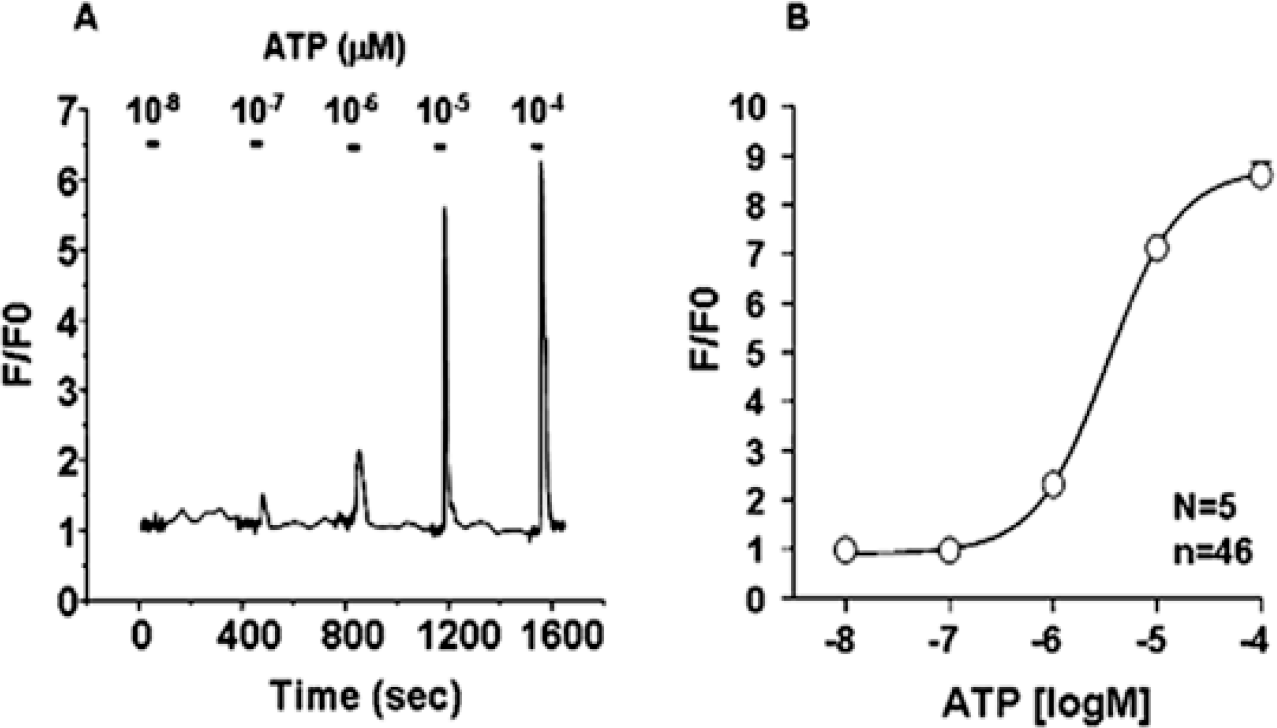
Fig. 2.
Concentration-response curve of P2Y receptor agonists in WERI-Rb-1 cells. Each curve shows the peak calcium responses of increasing concentrations of 2MeS-ATP, a selective P2Y1 agonist, αβ-MeATP, a P2X agonist, and UTP, a P2Y2/P2Y4/P2Y6 agonist. Both αβ-MeATP and UTP did not induce calcium rise. Values are means±S.E.M. n=total cell number, N=number of experiments.
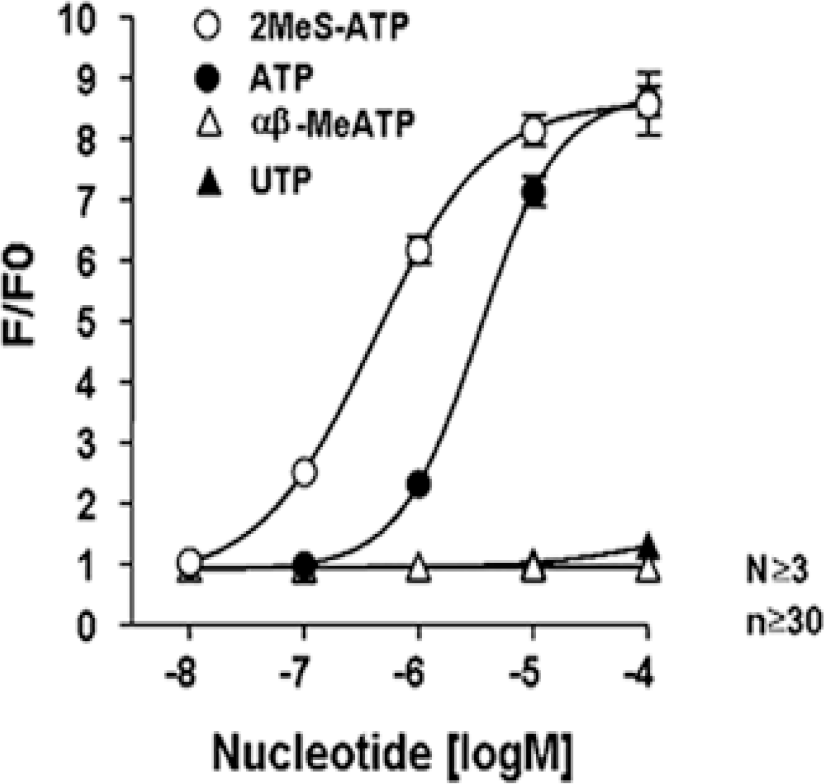
Fig. 3.
Differential effect of P2Y agonists on [Ca2+]i in WERI-Rb-1 cells. Original traces (A) and graph (B) showing calcium transient evoked by ATP (10 μM), a putative P2Y agonist, and its suppression after application of 30 μM MRS2179, a selective P2Y1 antagonist. F0: basal fluorescence after Fluo 3-AM loading, F: changes in fluorescence after introducing agonist or antagonist, n=total cell number, N=number of experiment. ∗∗∗Denotes p<0.001.
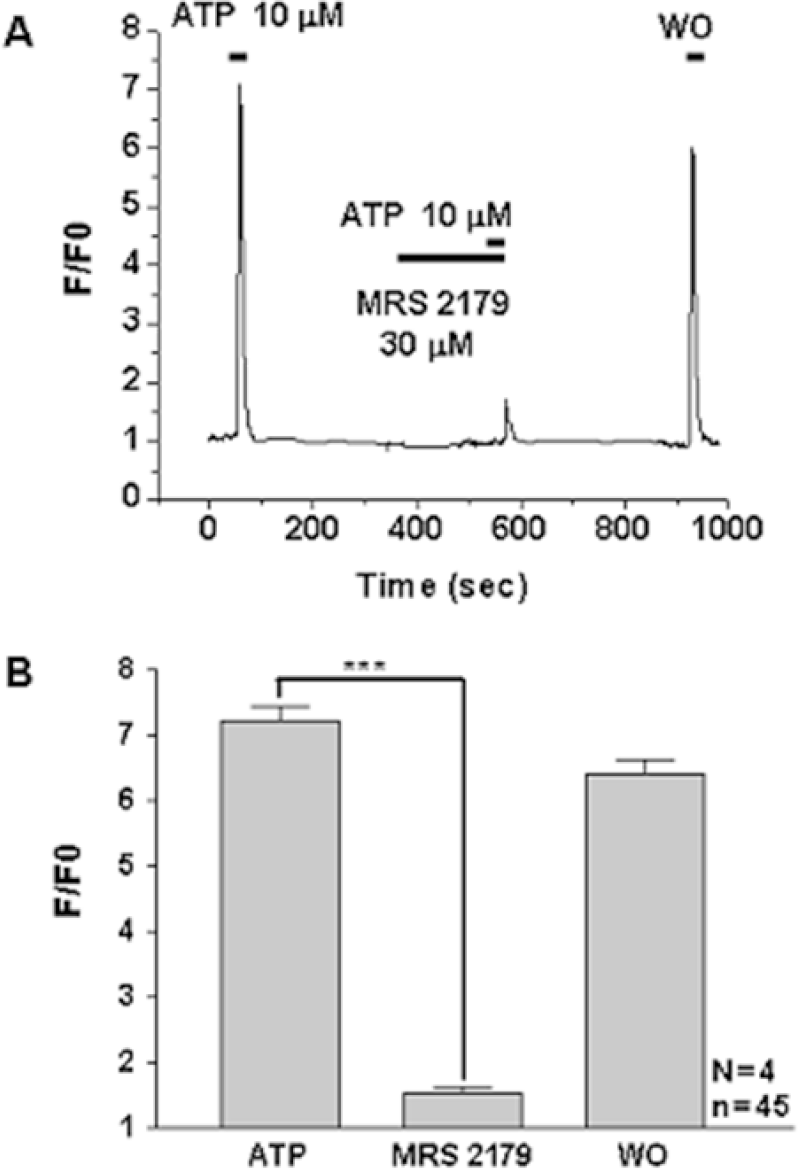
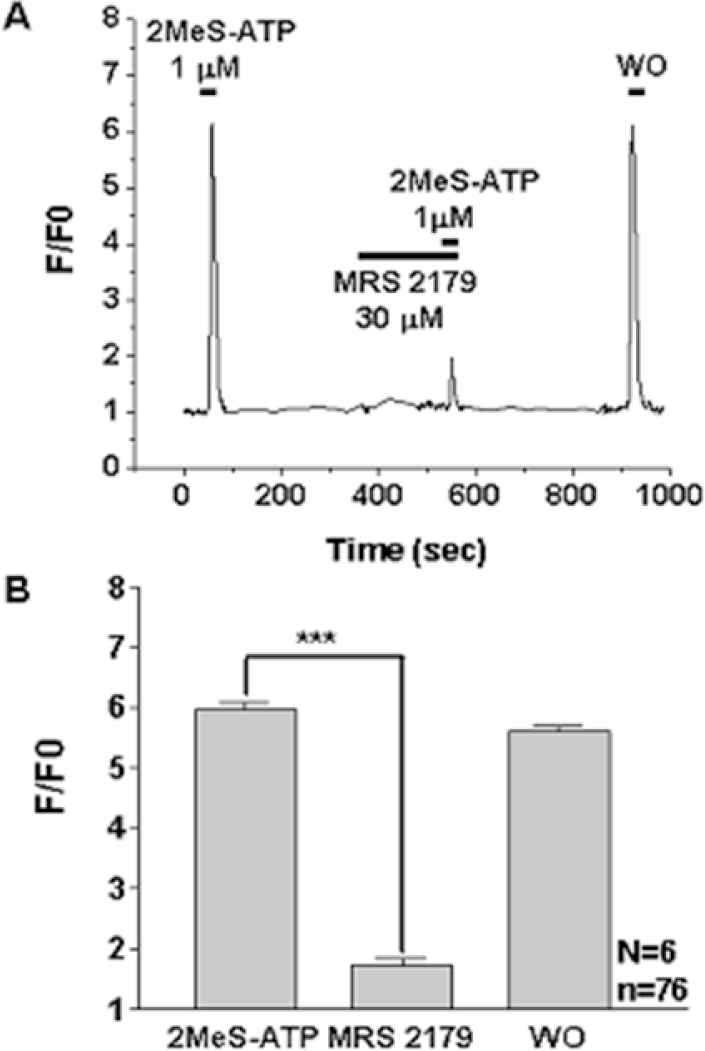
Fig. 4.
Effect of P2Y1 antagonist on 2-MeS-ATP-induced [Ca2+]i changes in WERI-Rb-1 cells. Original traces (A) and graph (B) showing calcium transient evoked by 2-MeS-ATP (1 μM), a P2Y1 agonist, and its suppression after application of the 30 μM MRS2179, a selective P2Y1 antagonist. F0: basal fluorescence after Fluo 3-AM loading, F: changes in fluorescence after introducing agonist or antagonist, n=total cell number, N=number of experiment, ∗∗∗denotes p<0.001.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download