Abstract
The secretion of insulin from pancreatic β-cells is triggered by the influx of Ca2+ through voltage-dependent Ca2+ channels. The resulting elevation of intracellular calcium ([Ca2+]i) triggers additional Ca2+ release from internal stores. Less well understood are the mechanisms involved in Ca2+ mobilization from internal stores after activation of Ca2+ influx. The mobilization process is known as calcium-induced calcium release (CICR). In this study, our goal was to investigate the existence of and the role of caffeine-sensitive ryanodine receptors (RyRs) in a rat pancreatic β-cell line, INS-1 cells. To measure cytosolic and stored Ca2+, respectively, cultured INS-1 cells were loaded with fura-2/AM or furaptra/AM. [Ca2+]i was repetitively increased by caffeine stimulation in normal Ca2+ buffer. However, peak [Ca2+]i was only observed after the first caffeine stimulation in Ca2+ free buffer and this increase was markedly blocked by ruthenium red, a RyR blocker. KCl-induced elevations in [Ca2+]i were reduced by pretreatment with ruthenium red, as well as by depletion of internal Ca2+ stores using cyclopiazonic acid (CPA) or caffeine. Caffeine-induced Ca2+ mobilization ceased after the internal stores were depleted by carbamylcholine (CCh) or CPA. In permeabilized INS-1 cells, Ca2+ release from internal stores was activated by caffeine, Ca2+, or ryanodine. Furthermore, ruthenium red completely blocked the CICR response in permeabilized cells. RyRs were widely distributed throughout the intracellular compartment of INS-1 cells. These results suggest that caffeine-sensitive RyRs exist and modulate the CICR response from internal stores in INS-1 pancreatic β-cells.
References
2. Hiriart M, Aguilar-Bryan L. Channel regulation of glucose sensing in the pancreatic β-cell. Am J Physiol Endocrinol Metab. 2008; 295:E1298–E1306.
3. Mears D. Regulation of insulin secretion in islets of Langerhans by Ca2+ channels. J Membr Biol. 2004; 200:57–66.
4. Varadi A, Rutter GA. Ca2+-induced Ca2+ release in pancreatic islet β-cells: critical evaluation of the use of endoplasmic reticulum-targeted “cameleons”. Endocrinology. 2004; 145:4540–4549.
5. Lemmens R, Larsson O, Berggren PO, Islam MS. Ca2+-induced Ca2+ release from the endoplasmic reticulum amplifies the Ca2+ signal mediated by activation of voltage-gated L-type Ca2+ channels in pancreatic β-cells. J Biol Chem. 2001; 276:9971–9977.
6. Graves TK, Hinkle PM. Ca2+-induced Ca2+ release in the pancreatic β-cell: direct evidence of endoplasmic reticulum Ca2+ release. Endocrinology. 2003; 144:3565–3574.
7. Hagar RE, Ehrlich BE. Regulation of the type III InsP3 receptor and its role in β cell function. Cell Mol Life Sci. 2000; 57:1938–1949.
8. Dyachok O, Tufveson G, Gylfe E. Ca2+-induced Ca2+ release by activation of inositol 1,4,5-trisphosphate receptors in primary pancreatic β-cells. Cell Calcium. 2004; 36:1–9.
9. Johnson JD, Kuang S, Misler S, Polonsky KS. Ryanodine receptors in human pancreatic β cells: localization and effects on insulin secretion. FASEB J. 2004; 18:878–880.
10. Bruton JD, Lemmens R, Shi CL, Persson-Sjögren S, Westerblad H, Ahmed M, Pyne NJ, Frame M, Furman BL, Islam MS. Ryanodine receptors of pancreatic β-cells mediate a distinct context-dependent signal for insulin secretion. FASEB J. 2003; 17:301–303.
11. Islam MS. The ryanodine receptor calcium channel of β-cells: molecular regulation and physiological significance. Diabetes. 2002; 51:1299–1309.
12. Kim BJ, Park KH, Yim CY, Takasawa S, Okamoto H, Im MJ, Kim UH. Generation of nicotinic acid adenine dinucleotide phosphate and cyclic ADP-ribose by glucagon-like peptide-1 evokes Ca2+ signal that is essential for insulin secretion in mouse pancreatic islets. Diabetes. 2008; 57:868–878.
13. Johnson JD, Misler S. Nicotinic acid-adenine dinucleotide phosphate-sensitive calcium stores initiate insulin signaling in human β cells. Proc Natl Acad Sci U S A. 2002; 99:14566–14571.
14. Islam MS, Larsson O, Nilsson T, Berggren PO. Effects of caffeine on cytoplasmic free Ca2+ concentration in pancreatic β-cells are mediated by interaction with ATP-sensitive K+ channels and L-type voltage-gated Ca2+ channels but not the ryanodine receptor. Biochem J. 1995; 306:679–686.
15. Rutter GA, Theler JM, Li G, Wollheim CB. Ca2+ stores in insulin-secreting cells: lack of effect of cADP ribose. Cell Calcium. 1994; 16:71–80.
16. Chen TH, Lee B, Yang C, Hsu WH. Effects of caffeine on intracellular calcium release and calcium influx in a clonal β-cell line RINm5F. Life Sci. 1996; 58:983–990.
17. Gamberucci A, Fulceri R, Pralong W, Bánhegyi G, Marcolongo P, Watkins SL, Benedetti A. Caffeine releases a glucose-primed endoplasmic reticulum Ca2+ pool in the insulin secreting cell line INS-1. FEBS Lett. 1999; 446:309–312.
18. Dror V, Kalynyak TB, Bychkivska Y, Frey MH, Tee M, Jeffrey KD, Nguyen V, Luciani DS, Johnson JD. Glucose and endoplasmic reticulum calcium channels regulate HIF-1β via presenilin in pancreatic β-cells. J Biol Chem. 2008; 283:9909–9916.
19. Takasawa S, Kuroki M, Nata K, Noguchi N, Ikeda T, Yamauchi A, Ota H, Itaya-Hironaka A, Sakuramoto-Tsuchida S, Takahashi I, Yoshikawa T, Shimosegawa T, Okamoto H. A novel ryanodine receptor expressed in pancreatic islets by alternative splicing from type 2 ryanodine receptor gene. Biochem Biophys Res Commun. 2010; 397:140–145.

20. Li F, Zhang ZM. Comparative identification of Ca2+ channel expression in INS-1 and rat pancreatic β cells. World J Gastroenterol. 2009; 15:3046–3050.
21. Park HS, Betzenhauser MJ, Won JH, Chen J, Yule DI. The type 2 inositol (1,4,5)-trisphosphate (InsP3) receptor determines the sensitivity of InsP3-induced Ca2+ release to ATP in pancreatic acinar cells. J Biol Chem. 2008; 283:26081–26088.
22. Choi KJ, Kim KS, Kim SH, Kim DK, Park HS. Caffeine and 2-aminoethoxydiphenyl borate (2-APB) have different ability to inhibit intracellular calcium mobilization in pancreatic acinar cell. Korean J Physiol Pharmacol. 2010; 14:105–111.

23. Rousseau E, Ladine J, Liu QY, Meissner G. Activation of the Ca2+ release channel of skeletal muscle sarcoplasmic reticulum by caffeine and related compounds. Arch Biochem Biophys. 1988; 267:75–86.
25. Ehrlich BE, Kaftan E, Bezprozvannaya S, Bezprozvanny I. The pharmacology of intracellular Ca2+-release channels. Trends Pharmacol Sci. 1994; 15:145–149.
26. Zalk R, Lehnart SE, Marks AR. Modulation of the ryanodine receptor and intracellular calcium. Annu Rev Biochem. 2007; 76:367–385.

27. Drews G, Krippeit-Drews P, Düfer M. Electrophysiology of islet cells. Adv Exp Med Biol. 2010; 654:115–163.

28. Braun M, Ramracheya R, Bengtsson M, Zhang Q, Karanauskaite J, Partridge C, Johnson PR, Rorsman P. Voltage-gated ion channels in human pancreatic β-cells: electrophysiological characterization and role in insulin secretion. Diabetes. 2008; 57:1618–1628.
29. Kang G, Holz GG. Amplification of exocytosis by Ca2+-induced Ca2+ release in INS-1 pancreatic β cells. J Physiol. 2003; 546:175–189.
30. Dyachok O, Gylfe E. Ca2+-induced Ca2+ release via inositol 1,4,5-trisphosphate receptors is amplified by protein kinase A and triggers exocytosis in pancreatic β-cells. J Biol Chem. 2004; 279:45455–45461.
31. Foskett JK, White C, Cheung KH, Mak DO. Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev. 2007; 87:593–658.
32. Islam MS, Larsson O, Berggren PO. Cyclic ADP-ribose in β cells. Science. 1993; 262:584–586.
33. Islam MS, Rorsman P, Berggren PO. Ca2+-induced Ca2+ release in insulin-secreting cells. FEBS Lett. 1992; 296:287–291.
34. Takasawa S, Nata K, Yonekura H, Okamoto H. Cyclic ADP-ribose in insulin secretion from pancreatic β cells. Science. 1993; 259:370–373.
35. Mitchell KJ, Lai FA, Rutter GA. Ryanodine receptor type I and nicotinic acid adenine dinucleotide phosphate receptors mediate Ca2+ release from insulin-containing vesicles in living pancreatic β-cells (MIN6). J Biol Chem. 2003; 278:11057–11064.
Fig. 1.
Caffeine stimulated calcium mobilization from internal stores in intact INS-1 cells. The representative traces show the effects of repetitive 30 mM caffeine stimulation on [Ca2+]i increases in the presence (A) and absence (B) of extracellular Ca2+. The data were obtained from 5 and 7 separate experiments, respectively. INS-1 cells were responsive to repetitive caffeine stimulation in normal extracellular Ca2+ buffer, but only responded to the first caffeine stimulation in Ca2+ free solution. (C) A 50 μM of ruthenium red markedly reduced the [Ca2+]i peak in the absence of extracellular Ca2+. Data were normalized to control values and expressed as mean %±S.E. Asterisk indicates the value is significantly different from the corresponding value of caffeine alone (p<0.05).
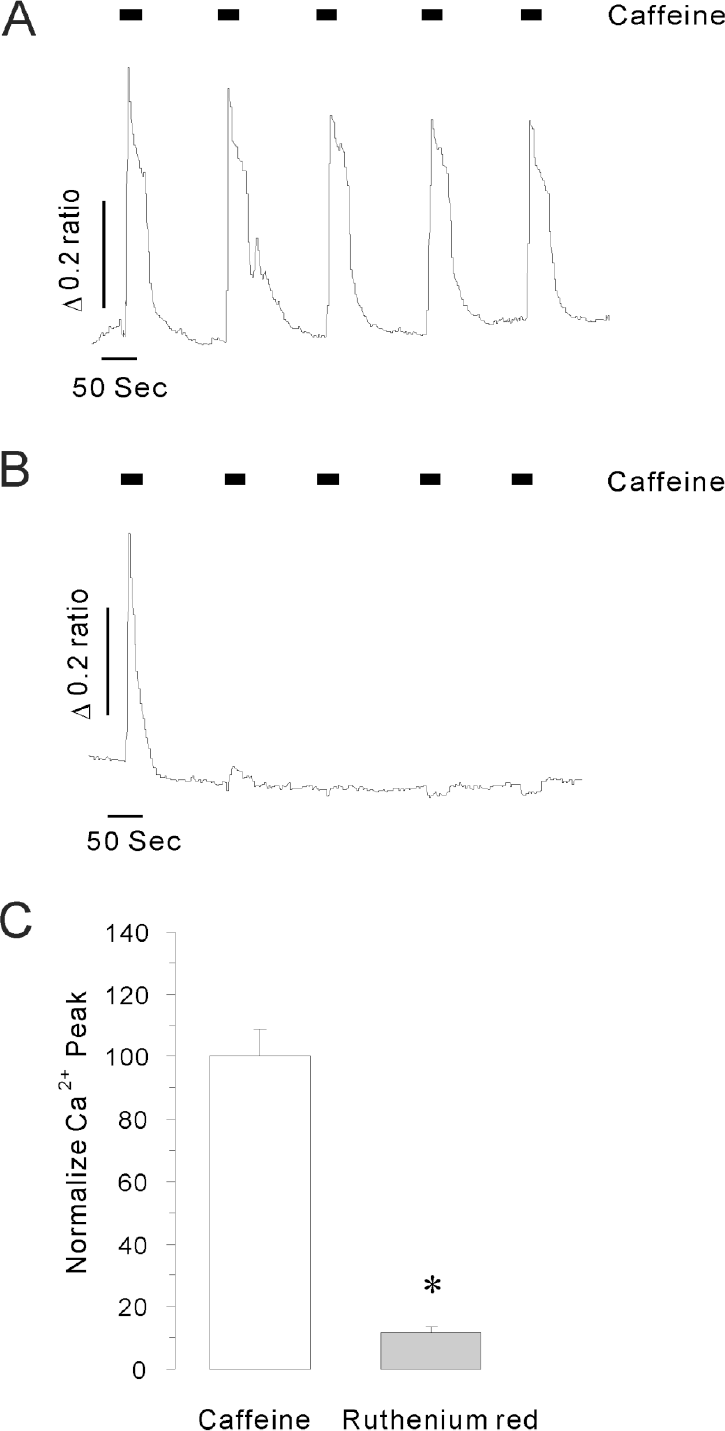
Fig. 2.
KCl triggered Ca2+ release from internal stores in intact INS-1 cells. (A) The representative trace shows the effect of 45 mM KCl on [Ca2+]i increases in the presence and absence of extracellular Ca2+. The data were obtained from 6 separate experiments. [Ca2+]i elevation was not observed in Ca2+ free medium. (B) Effects of CPA plus caffeine, CPA alone or caffeine alone on KCl-induced [Ca2+]i peaks in the presence of extracellular Ca2+. The data were obtained from at least 5 separate experiments. Data were normalized to the initial [Ca2+]i peak and expressed as mean % ± S.E. Asterisks indicate that the values are significantly different from the corresponding value for control (p<0.05). Intracellular Ca2+ store depletion reduced depolarization-induced Ca2+ mobilization. (C) Representative trace shows the effect of ruthenium red on KCl-induced [Ca2+]i elevations. The data were obtained from 6 separate experiments. A 50 μM of ruthenium red significantly reduced depolarization-induced Ca2+ mobilization; the effect was restored after washout of the ruthenium red.
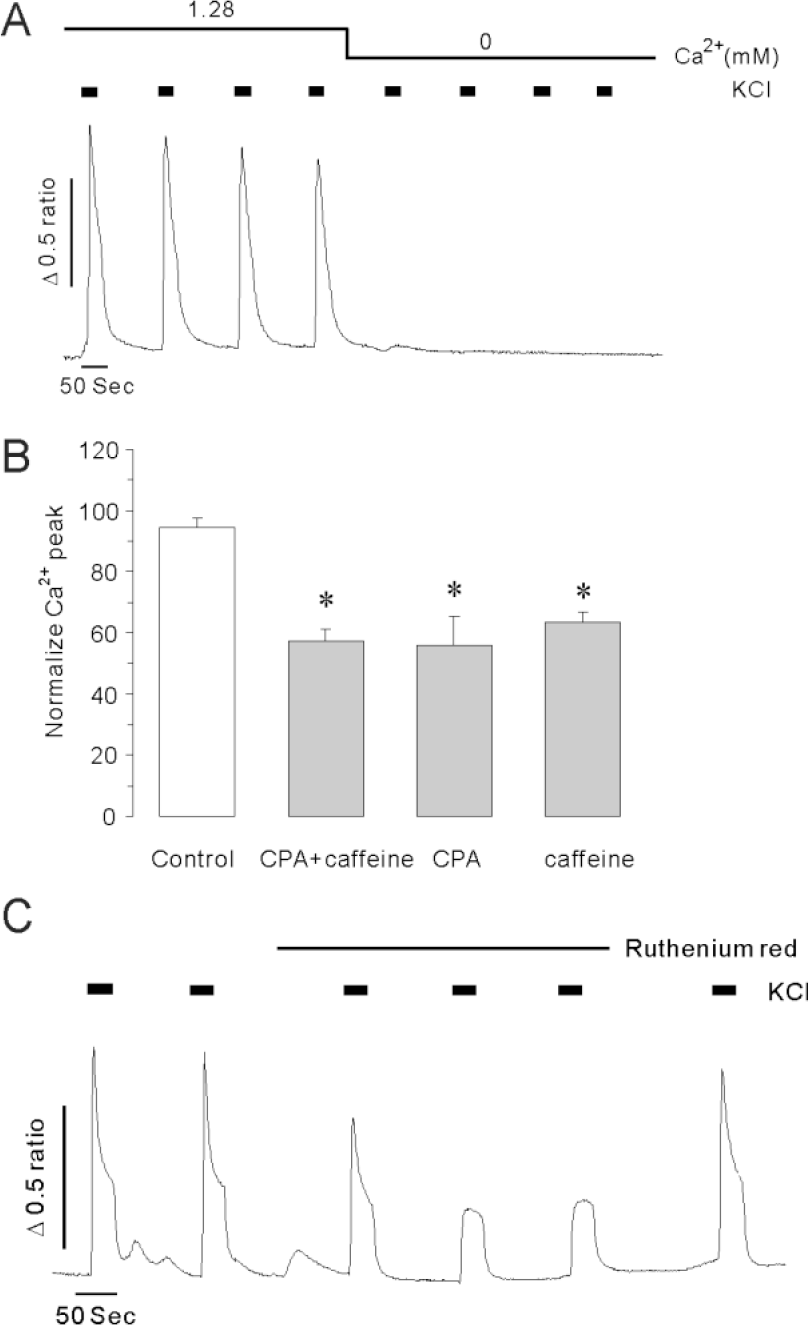
Fig. 3.
Effects of internal calcium store depletion on caffeine-induced calcium release. (A) The representative trace shows the 30 mM caffeine-induced [Ca2+]i rise after internal store depletion by 10 μM carbamylcholine (CCh) in Ca2+ free solution. (B) The representative trace shows the 10 μM CCh-induced [Ca2+]i rise under store depleted conditions induced by pretreatment with 30 mM caffeine in the absence of extracellular Ca2+. (C) A representative trace of the caffeine effect under store depleted condition induced by pretreatment of cells with 10 μM cyclopiazonic acid (CPA) in Ca2+ free solution. All data were obtained from at least 5 separate experiments. Caffeine failed to increase [Ca2+]i after internal Ca2+ store depletion induced by pretreatment with CCh or CPA.
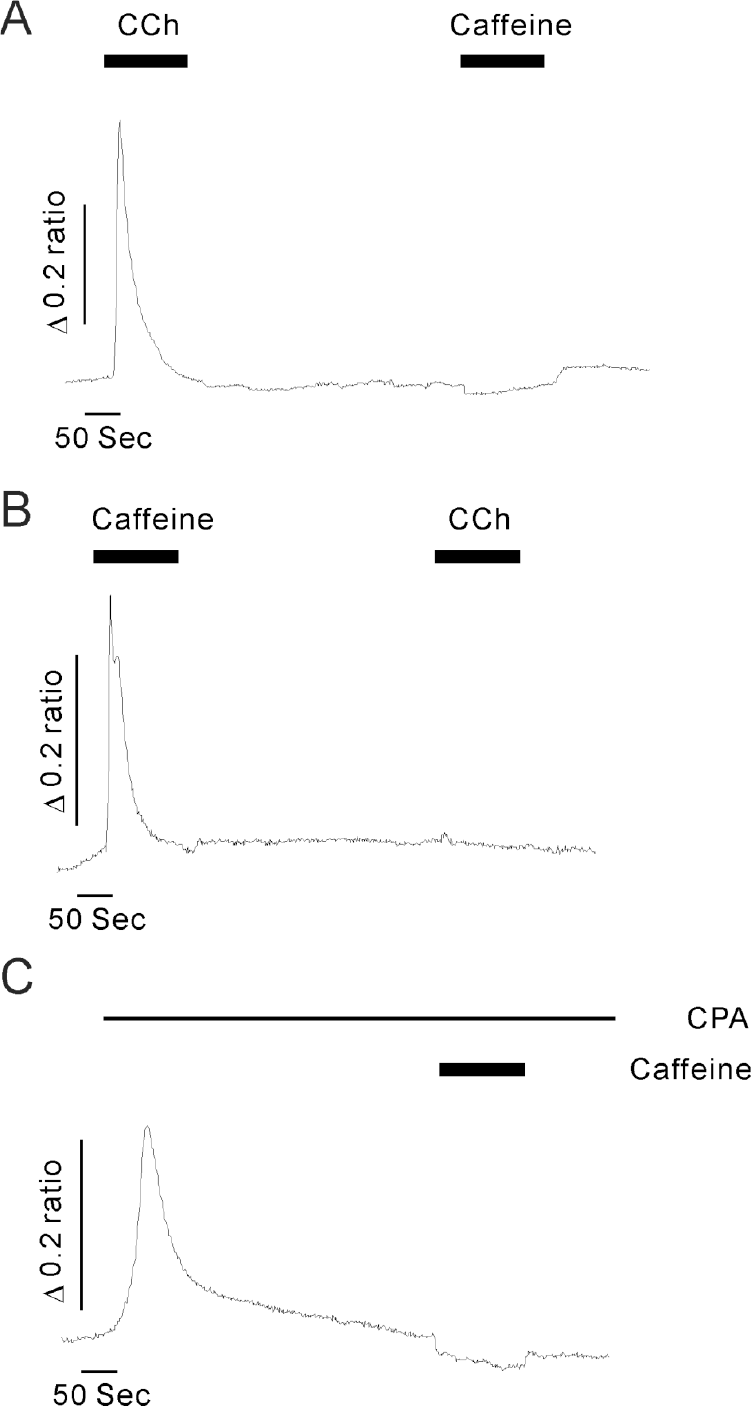
Fig. 4.
Caffeine, Ca2+ or ryanodine-induced calcium release in permeabilized INS-1 cells. (A) 10 mM caffeine (☐), 10 μM Ca2+ (⋄) or 1 μM ryanodine (Δ) significantly stimulated Ca2+ release from internal stores in permeabilized INS-1 cells. Arrows indicate the starting point of each drug perfusion. (B) Summarized Ca2+ release rates (S–1) induced by caffeine, Ca2+ or ryanodine. Data were summarized from at least 5 experiments. Asterisks indicate that the values are significantly different from the corresponding value for control (p<0.05). (C) The blocking effect of 50 μM ruthenium red on 10 μM Ca2+-induced Ca2+ release in permeabilized INS-1 cells. (D) Summarized data showing the effects of ruthenium red on Ca2+-induced Ca2+ release rates in permeabilized cells. Asterisk indicates that the value is significantly different from the corresponding value of Ca2+ (p<0.05). Ca2+ release induced by elevated Ca2+ was completely blocked by ruthenium red, a RyR blocker, in permeabilized INS-1 cells.
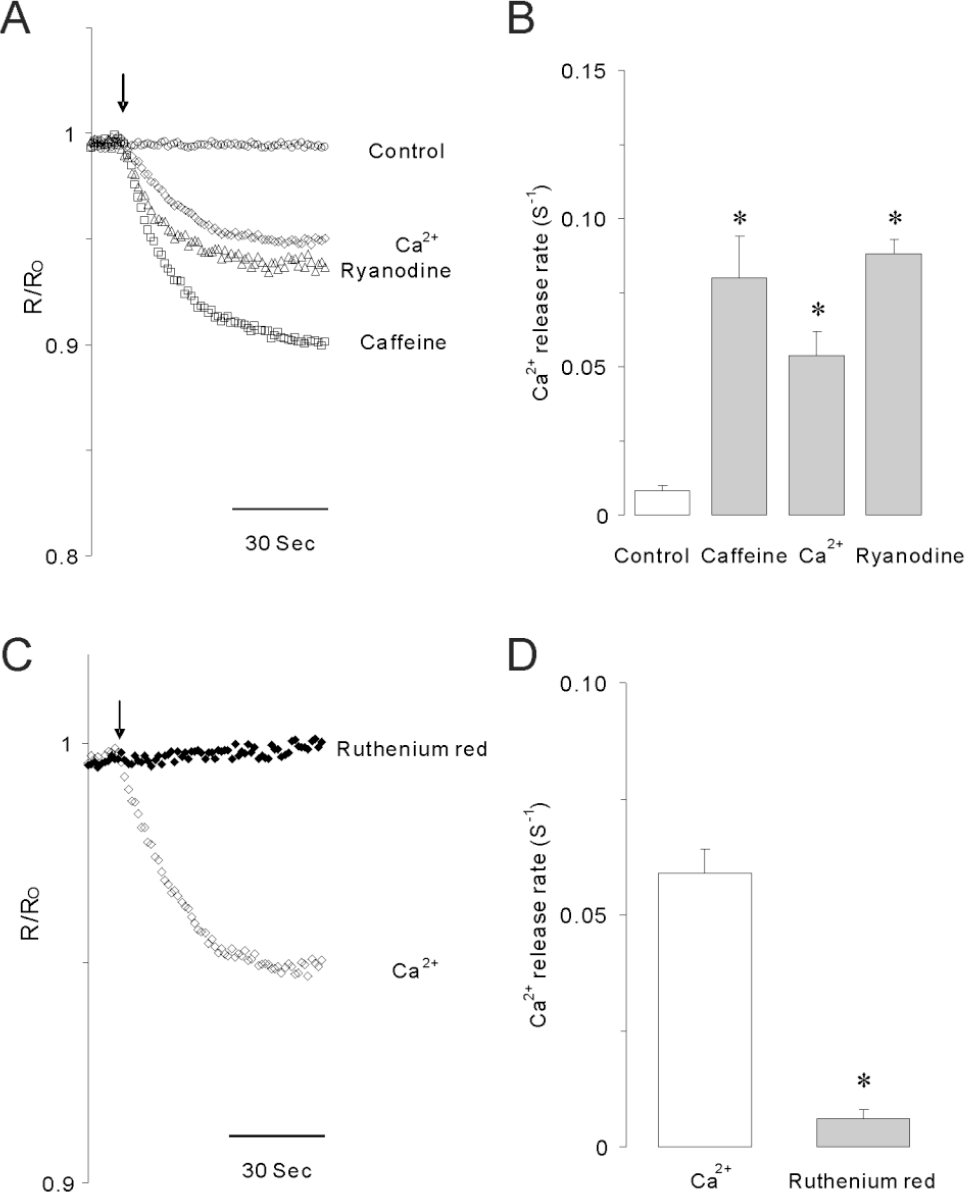
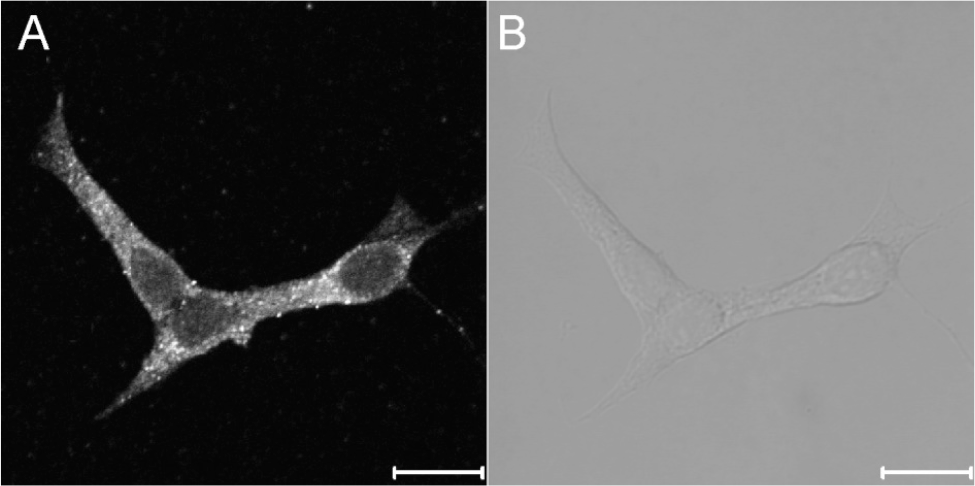
Fig. 5.
The expression of ryanodine receptors in INS-1 rat insulinoma cells. The fluorescence image (A) and the bright image (B) show the expression and distribution of RyRs in the intracellular compartments. The images were obtained from 4 separate experiments. Immunocytochemistry was done using primary RyR antibody as described under experimental procedures. The scale bar is 10 μm.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download