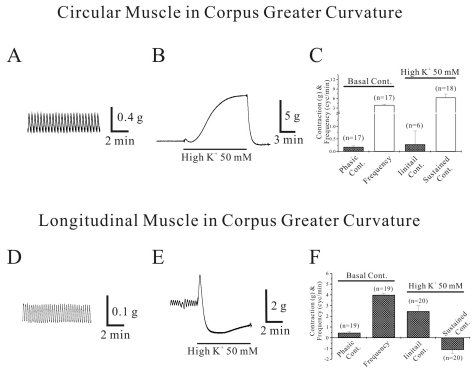
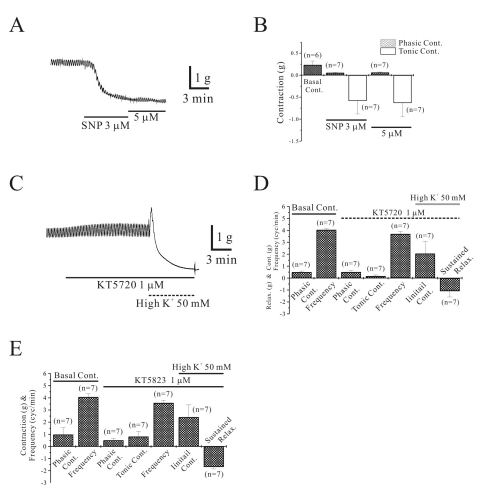
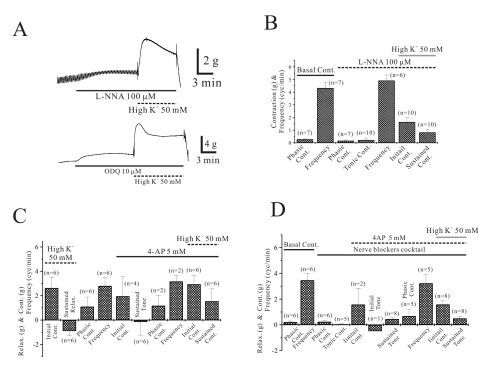
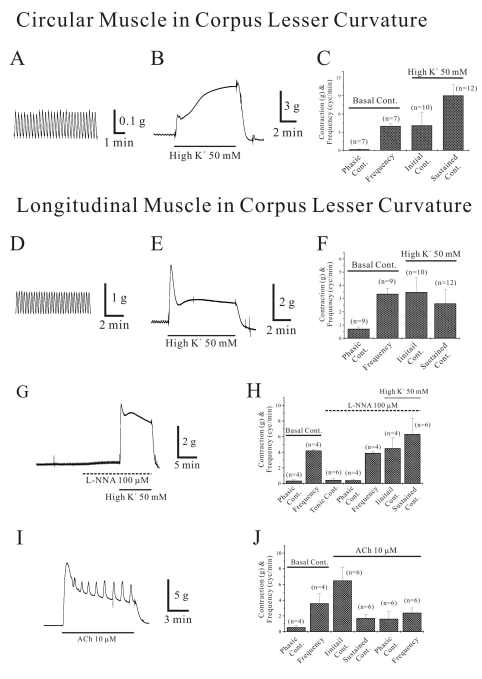
1. Schubert ML, Makhlouf GM. Gastrin secretion induced by distention is mediated by gastric cholinergic and vasoactive intestinal peptide neurons in rats. Gastroenterology. 1993; 104:834–839. PMID:
8095036.

2. Shafik A, El Sibai O, Shafik AA, Shafik IA. Mechanism of gastric emptying through the pyloric sphincter: a human study. Med Sci Monit. 2007; 13:CR24–CR29. PMID:
17179906.
3. Hennig GW, Brookes SJ, Costa M. Excitatory and inhibitory motor reflexes in the isolated guinea-pig stomach. J Physiol. 1997; 501:197–212. PMID:
9175003.

4. Cannon WB, Lieb CW. The receptive relaxation of the stomach. Am J Physiol. 1911; 29:267–273.

5. Paton WD, Vane JR. Analysis of he responses of the isolated stomach to electrical stimulation and to drugs. J Physiol. 1963; 165:10–46. PMID:
13941859.
6. Desai KM, Sessa WC, Vane JR. Involvement of nitric oxide in the reflex relaxation of the stomach to accommodate food or fluid. Nature. 1991; 351:477–479. PMID:
1675430.

7. Abrahamsson H. Studies on the inhibitory nervous control of gastric motility. Acta Physiol Scand Suppl. 1973; 390:1–38. PMID:
4521069.
8. Barbier AJ, Lefebvre RA. Involvement of the L-arginine: nitric oxide pathway in nonadrenergic noncholinergic relaxation of the cat gastric fundus. J Pharmacol Exp Ther. 1993; 266:172–178. PMID:
8331556.
9. Lefebvre RA, Smits GJ, Timmermans JP. Study of NO and VIP as non-adrenergic non-cholinergic neurotransmitters in the pig gastric fundus. Br J Pharmacol. 1995; 116:2017–2026. PMID:
8640340.

10. Lefebvre RA, Baert E, Barbier AJ. Influence of NG-nitro-L-arginine on non-adrenergic non-cholinergic relaxation in the guinea-pig gastric fundus. Br J Pharmacol. 1992; 106:173–179. PMID:
1504726.

11. Yano S, Kiyota Y, Yamamoto M, Watanabe K. Pharmacological features of non-adrenergic non-cholinergic (NANC) relaxation induced by electrical vagal stimulation in isolated mouse stomach. Jpn J Pharmacol. 1995; 69:9–15. PMID:
8847835.

12. Tonini M, De Giorgio R, De Ponti F, Sternini C, Spelta V, Dionigi P, Barbara G, Stanghellini V, Corinaldesi R. Role of nitric oxide- and vasoactive intestinal polypeptide-containing neurones in human gastric fundus strip relaxations. Br J Pharmacol. 2000; 129:12–20. PMID:
10694197.

13. Cousins HM, Edwards FR, Hickey H, Hill CE, Hirst GD. Electrical coupling between the myenteric interstitial cells of Cajal and adjacent muscle layers in the guinea-pig gastric antrum. J Physiol. 2003; 550:829–844. PMID:
12844505.

14. Kuriyama H, Mishima K, Suzuki H. Some differences in contractile responses of isolated longitudinal and circular muscle from the guinea-pig stomach. J Physiol. 1975; 251:317–331. PMID:
1185667.

15. Osa T, Kuriyama H. The membrane properties and decremental conduction of excitation in the fundus of the guinea-pig stomach. Jpn J Physiol. 1970; 20:626–639. PMID:
5313777.

16. Connor C, Prosser CL. Comparison of ionic effects on longitudinal and circular muscle of cat jejunum. Am J Physiol. 1974; 226:1212–1218. PMID:
4824873.

17. Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature. 1994; 372:231–236. PMID:
7969467.

18. Hagiwara S, Byerly L. Membrane biophysics of calcium currents. Fed Proc. 1981; 40:2220–2225. PMID:
7238906.
19. Kim YC, Choi W, Sung R, Kim H, You RY, Park SM, Youn SJ, Kim MJ, Song YJ, Xu WX, Lee SJ, Yun HY. Relaxation patterns of human gastric corporal smooth muscle by cyclic nucleotides producing agents. Korean J Physiol Pharmacol. 2009; 13:503–510. PMID:
20054499.

20. Kim SJ, Ahn SC, Kim JK, Kim YC, So I, Kim KW. Changes in intracellular Ca
2+ concentration induced by L-type Ca
2+ channel current in guinea pig gastric myocytes. Am J Physiol. 1997; 273:C1947–C1956. PMID:
9435500.
21. Barajas-López C, Huizinga JD. Different mechanisms of contraction generation in circular muscle of canine colon. Am J Physiol. 1989; 256:G570–G580. PMID:
2923215.
22. Kelly KA, Code CF. Canine gastric pacemaker. Am J Physiol. 1971; 220:112–118. PMID:
5538644.

23. Xu WX, Kim SJ, So I, Kim KW. Role of actin microfilament in osmotic stretch-induced increase of voltage-operated calcium channel current in guinea-pig gastric myocytes. Pflugers Arch. 1997; 434:502–504. PMID:
9211820.

24. Xu WX, Li Y, Wu LR, Li ZL. Effects of different kinds of stretch on voltage-dependent calcium current in antrial circular smooth muscle cells of the guinea-pig. Sheng Li Xue Bao. 2000; 52:69–74. PMID:
11971175.
25. Burnstock G. The non-adrenergic non-cholinergic nervous system. Arch Int Pharmacodyn Ther. 1986; 280(2 Suppl):1–15. PMID:
3015062.
26. Dixit D, Zarate N, Liu LW, Boreham DR, Huizinga JD. Interstitial cells of Cajal and adaptive relaxation in the mouse stomach. Am J Physiol Gastrointest Liver Physiol. 2006; 291:G1129–G1136. PMID:
16891301.

27. Park WS, Ko JH, Ko EA, Son YK, Hong da H, Jung ID, Park YM, Choi TH, Kim N, Han J. The guanylyl cyclase activator YC-1 directly inhibits the voltage-dependent K
+ channels in rabbit coronary arterial smooth muscle cells. J Pharmacol Sci. 2010; 112:64–72. PMID:
20093789.
28. Yuan XJ, Tod ML, Rubin LJ, Blaustein MP. NO hyperpolarizes pulmonary artery smooth muscle cells and decreases the intracellular Ca
2+ concentration by activating voltage-gated K
+ channels. Proc Natl Acad Sci USA. 1996; 93:10489–10494. PMID:
8816828.
29. Yu YC, Guo HS, Li Y, Piao L, Li L, Li ZL, Xu WX. Role of calcium mobilization in sodium nitroprusside-induced increase of calcium-activated potassium currents in gastric antral circular myocytes of guinea pig. Acta Pharmacol Sin. 2003; 24:819–825. PMID:
12904283.
30. Ibba Manneschi L, Pacini S, Corsani L, Bechi P, Faussone-Pellegrini MS. Interstitital cells of Cajal in the human stomach: distribution and relationship with enteric innervation. Histol Histopathol. 2004; 19:1153–1164. PMID:
15375758.
31. Iino S, Horiguchi K, Nojyo Y. Interstitial cells of Cajal are innervated by nitrergic nerves and express nitric oxide-sensitive guanylate cyclase in the guinea-pig gastrointestinal tract. Neuroscience. 2008; 152:437–448. PMID:
18280665.

32. Nemeth L, Puri P. Three-dimensional morphology of c-
Kit-positive cellular network and nitrergic innervation in the human gut. Arch Pathol Lab Med. 2001; 125:899–904. PMID:
11419974.
33. Kim YC, Suzuki H, Xu WX, Hashitani H, Choi W, Yun HY, Park SM, Youn SJ, Lee SJ, Lee SJ. Voltage-dependent Ca2+ current identified in freshly isolated interstitial cells of cajal (ICC) of guinea-pig stomach. Korean J Physiol Pharmacol. 2008; 12:323–330. PMID:
19967074.
34. Kim YC, Suzuki H, Xu WX, Choi W, Kim SH, Lee SJ. Ca
2+-activated K
+ current in freshly isolated c-
Kit positive cells in guinea-pig stomach. J Korean Med Sci. 2009; 24:384–391. PMID:
19543421.
35. Sung R, Kim YC, Yun HY, Choi W, Kim HS, Kim H, Lee KJ, You RY, Park SM, Youn SJ, Kim MJ, Kim WS, Song YJ, Kim SY, Xu WX, Lee SJ. Interstitial cells of Cajal (ICC)-like-c-
Kit positive cells are involved in gastritis and carcinogenesis in human stomach. Oncol Rep. 2011; 26:33–42. PMID:
21573494.
36. Yun HY, Sung R, Kim YC, Choi W, Kim HS, Kim H, Lee GJ, You RY, Park SM, Yun SJ, Kim MJ, Kim WS, Song YJ, Xu WX, Lee SJ. Regional distribution of interstitial cells of cajal (ICC) in human stomach. Korean J Physiol Pharmacol. 2010; 14:317–324. PMID:
21165331.