Abstract
We have shown that myosin light chain kinase (MLCK) was required for the off-contraction in response to the electrical field stimulation (EFS) of feline esophageal smooth muscle. In this study, we investigated whether protein kinase C (PKC) may require the on-contraction in response to EFS using feline esophageal smooth muscle. The contractions were recorded using an isometric force transducer. On-contraction occurred in the presence of Ng-nitro-L-arginine methyl ester (L-NAME), suggesting that nitric oxide acts as an inhibitory mediator in smooth muscle. The excitatory composition of both contractions was cholinergic dependent which was blocked by tetrodotoxin or atropine. The on-contraction was abolished in Ca2+-free buffer but reappeared in normal Ca2+-containing buffer indicating that the contraction was Ca2+ dependent. 4-aminopyridine (4-AP), voltage-dependent K+ channel blocker, significantly enhanced on-contraction. Aluminum fluoride (a G-protein activator) increased on-contraction. Pertussis toxin (a Gi inactivator) and C3 exoenzyme (a rhoA inactivator) significantly decreased on-contraction suggesting that Gi or rhoA protein may be related with Ca2+ and K+ channel. ML-9, a MLCK inhibitor, significantly inhibited on-contraction, and chelerythrine (PKC inhibitor) affected on the contraction. These results suggest that endogenous cholinergic contractions activated directly by low-frequency EFS may be mediated by Ca2+, and G proteins, such as Gi and rhoA, which resulted in the activation of MLCK, and PKC to produce the contraction in feline distal esophageal smooth muscle.
References
1. Conklin JL. Nitric oxide: a mediator of esophageal motor function. J Lab Clin Med. 1998; 131:10–20.

2. Preiksaitis HG, Tremblay L, Diamant NE. Nitric oxide mediates inhibitory nerve effects in human esophagus and lower esophageal sphincter. Dig Dis Sci. 1994; 39:770–775.

3. Richards WG, Sugarbaker DJ. Neuronal control of esophageal function. Chest Surg Clin N Am. 1995; 5:157–171.
4. Crist J, Gidda JS, Goyal RK. Intramural mechanism of esophageal peristalsis: Roles of cholinergic and noncholinergic nerves. Proc Natl Acad Sci U S A. 1984; 81:3595–3599.

5. Gilbert RJ, Dodds WJ. Effect of selective muscarinic antagonists on peristaltic contractions in opossum smooth muscle. Am J Physiol. 1986; 250:G50–59.

6. Jury J, Ahmedzadeh N, Daniel EE. A mediator derived from arginine mediates inhibitory junction potentials and relaxations in lower esophageal sphincter: An independent role for vasoactive intestinal peptide. Can J Physiol Pharmacol. 1992; 70:1182–1189.

7. Murray J, Du C, Ledlow A, Bates JN, Conklin JL. Nitric oxide: Mediator of nonadrenergic noncholinergic responses of opossum esophageal muscle. Am J Physiol. 1991; 261:G401–406.

8. Crist J, Gidda JS, Goyal RK. Characteristics of “On” And “Off” Contractions in esophageal circular muscle in vitro. Am J Physiol. 1984; 246:G137–144.

9. Hong KW, Biancani P, Weiss RM. “On” And “Off” Responses of guinea pig ureter. Am J Physiol. 1985; 248:C165–169.

10. Gonzalez AA, Farre R, Clave P. Different responsiveness of excitatory and inhibitory enteric motor neurons in the human esophagus to electrical field stimulation and to nicotine. Am J Physiol Gastrointest Liver Physiol. 2004; 287:G299–306.
11. Yamato S, Spechler SJ, Goyal RK. Role of nitric oxide in esophageal peristalsis in the opossum. Gastroenterology. 1992; 103:197–204.

12. Wade GR, Laurier LG, Preiksaitis HG, Sims SM. Delayed rectifier and Ca2+-dependent K+ currents in human esophagus: Roles in regulating muscle contraction. Am J Physiol. 1999; 277:G885–895.
13. Park JH, Kim HS, Park SY, Im C, Jeong JH, Kim IK, Sohn UD. The influences of g proteins, Ca, and K channels on electrical field stimulation in cat esophageal smooth muscle. Korean J Physiol Pharmacol. 2009; 13:393–400.
14. Holian O, Astumian RD, Lee RC, Reyes HM, Attar BM, Walter RJ. Protein kinase c activity is altered in hl60 cells exposed to 60 hz ac electric fields. Bioelectromagnetics. 1996; 17:504–509.

15. Marin J, Ferrer M, Balfagon G. Role of protein kinase c in electrical-stimulation-induced neuronal nitric oxide release in mesenteric arteries from hypertensive rats. Clin Sci (Lond). 2000; 99:277–283.
16. Park SY, Park SU, Sohn UD. Regulators involved in the electrically stimulated response of feline esophageal smooth muscle. Pharmacology. 2009; 84:346–355.

17. Yamboliev IA, Mutafova-Yambolieva VN. Pi3k and pkc contribute to membrane depolarization mediated by alpha2-adrenoceptors in the canine isolated mesenteric vein. BMC Physiol. 2005; 5:9.
18. Anschutz S, Schubert R. Modulation of the myogenic response by neurogenic influences in rat small arteries. Br J Pharmacol. 2005; 146:226–233.
19. Harnett KM, Cao W, Biancani P. Signal-transduction pathways that regulate smooth muscle function i. Signal transduction in phasic (esophageal) and tonic (gastroesophageal sphincter) smooth muscles. Am J Physiol Gastrointest Liver Physiol. 2005; 288:G407–416.

20. Jury J, Boev KR, Daniel EE. Nitric oxide mediates outward potassium currents in opossum esophageal circular smooth muscle. Am J Physiol. 1996; 270:G932–938.

21. Park SY, Song HJ, Sohn UD. Participation of rho-associated kinase in electrical stimulated and acetylcholine-induced contraction of feline esophageal smooth muscle. Eur J Pharmacol. 2009; 607:220–225.

22. Rae MG, Khoyi MA, Keef KD. Modulation of cholinergic neuromuscular transmission by nitric oxide in canine colonic circular smooth muscle. Am J Physiol. 1998; 275:G1324–1332.
23. Giovannini F, Sher E, Webster R, Boot J, Lang B. Calcium channel subtypes contributing to acetylcholine release from normal, 4-aminopyridine-treated and myasthenic syndrome auto-antibodies-affected neuromuscular junctions. Br J Pharmacol. 2002; 136:1135–1145.

24. Katz B, Miledi R. Further study of the role of calcium in synaptic transmission. J Physiol. 1970; 207:789–801.

25. Sohn UD, Chiu TT, Bitar KN, Hillemeier C, Behar J, Biancani P. Calcium requirements for acetylcholine-induced contraction of cat esophageal circular muscle cells. Am J Physiol. 1994; 266:G330–338.

26. Biancani P, Hillemeier C, Bitar KN, Makhlouf GM. Contraction mediated by Ca2+ influx in esophageal muscle and by Ca2+ release in the les. Am J Physiol. 1987; 253:G760–766.
27. Stull JT, Lin PJ, Krueger JK, Trewhella J, Zhi G. Myosin light chain kinase: Functional domains and structural motifs. Acta Physiol Scand. 1998; 164:471–482.

28. Kiaii B, Xu QW, Shaffer EA. The basis for progesterone impairment of gallbladder contractility in male guinea pigs in vitro. J Surg Res. 1998; 79:97–102.
29. Ratz PH, Blackmore PF. Differential activation of rabbit femoral arteries by aluminum fluoride and sodium fluoride. J Pharmacol Exp Ther. 1990; 254:514–520.
30. Sohn UD, Hong YW, Choi HC, Ha JH, Lee KY, Kim WJ, Biancani P, Jeong JH, Huh IH. Increase of [Ca2+]i and release of arachidonic acid via activation of m2 receptor coupled to gi and rho proteins in oesophageal muscle. Cell Signal. 2000; 12:215–222.
31. Daniel EE, Posey-Daniel V. Effects of scorpion venom on structure and function of esophageal lower sphincter (LES) and body circular muscle (BCM) from opossum. Can J Physiol Pharmacol. 1984; 62:360–373.

32. Daniel EE, Posey-Daniel V. Neuromuscular structures in opossum esophagus: Role of interstitial cells of cajal. Am J Physiol. 1984; 246:G305–315.

33. Yuan XJ. Voltage-gated K+ currents regulate resting membrane potential and [Ca2+]i in pulmonary arterial myocytes. Circ Res. 1995; 77:370–378.
34. Yuan XJ, Tod ML, Rubin LJ, Blaustein MP. No hyperpolarizes pulmonary artery smooth muscle cells and decreases the intracellular Ca2+ concentration by activating voltage-gated K+ channels. Proc Natl Acad Sci U S A. 1996; 93:10489–10494.
35. Zhang Y, Vogalis F, Goyal RK. Nitric oxide suppresses a Ca2+-stimulated Cl− current in smooth muscle cells of opossum esophagus. Am J Physiol. 1998; 274:G886–890.
Fig. 1.
Effect of external [Ca2+] on the on-contraction in the presence of L-NAME by EFS. (A) Representative trace of the effects of various external [Ca2+] on the on-contraction in the presence of 100 μM L-NAME (left). (B) The changes in amplitudes of the contraction affected by various external [Ca2+] were expressed as gram (g) (right). Results are expressed as means±S.E. of 4 experiments. ∗p<0.05, ∗∗p<0.01 compared with 0 mM external [Ca2+] for on-contraction in the presence of 100 μM L-NAME (ANOVA).
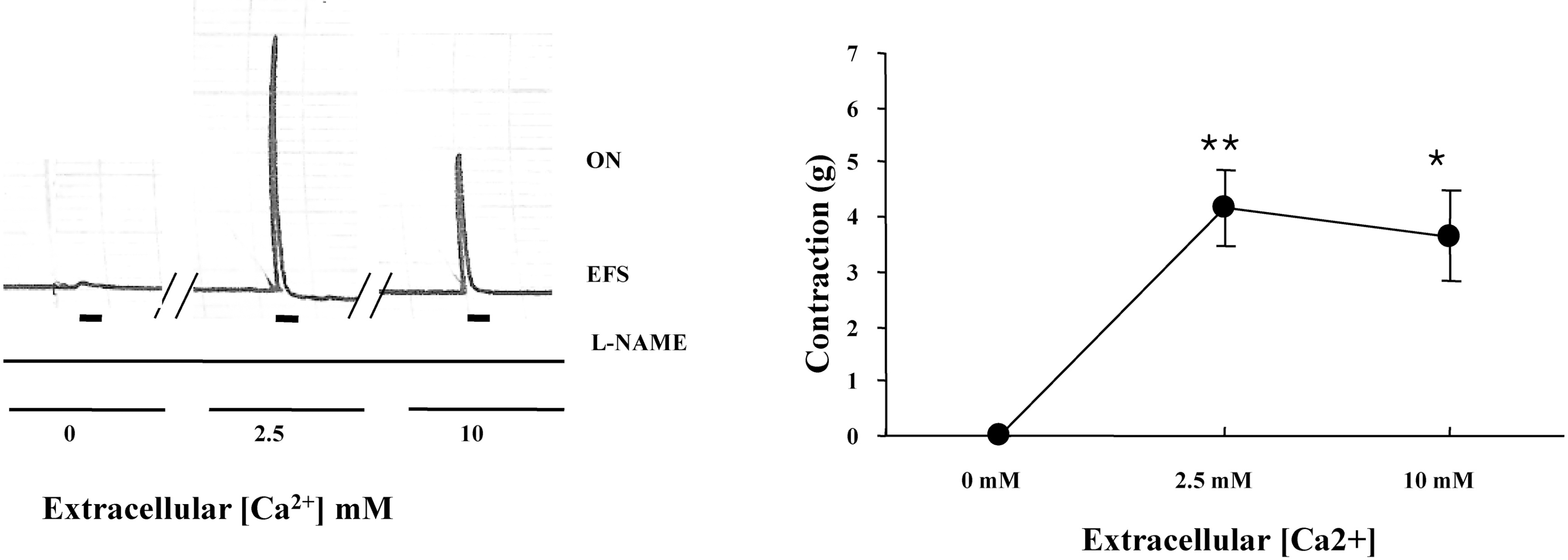
Fig. 2.
Effect of 4-AP on the on-contraction in the presence of L-NAME by EFS. The changes in amplitudes of the on-contractions affected by 4-AP were expressed as % of control. The contraction was significantly augmented by 4-AP. Results are expressed as means±S.E. of 6 experiments ∗p<0.05, ∗∗p<0.01 compared with control of on-contraction in the presence of L-NAME (ANOVA).
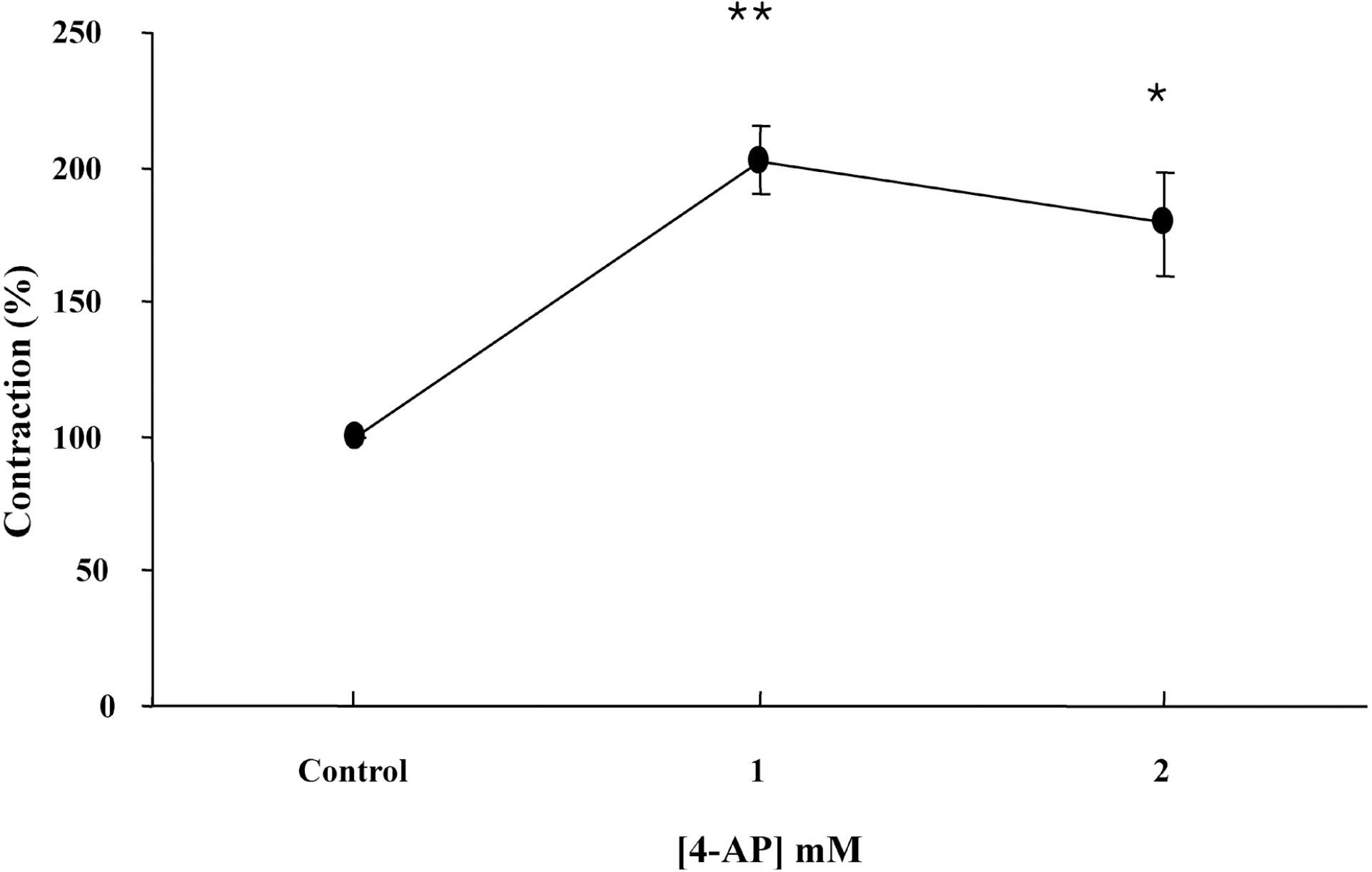
Fig. 3.
Effect of AlF the on-contraction in the presence of L-NAME. Muscle strips were incubated with AlF for 10 min or 30 min in various concentrations and then changes of EFS-induced contractile response in presence of L-NAME were measured. AlF increased the on-contraction in the presence of L-NAME in a concentration-dependent manner. Results are expressed as means±S.E. of 5 experiments. ∗p<0.05, ∗∗p<0.01, compared with control of on-contraction in the presence of L-NAME for set of 2 mM AlF, ##p<0.01 compared with control of on-contraction in the presence of L-NAME for set of 10 mM AlF(ANOVA), $p<0.05 compared with on-contraction at 30 min pretreatment with 2 mM of AlF (Student t-test).
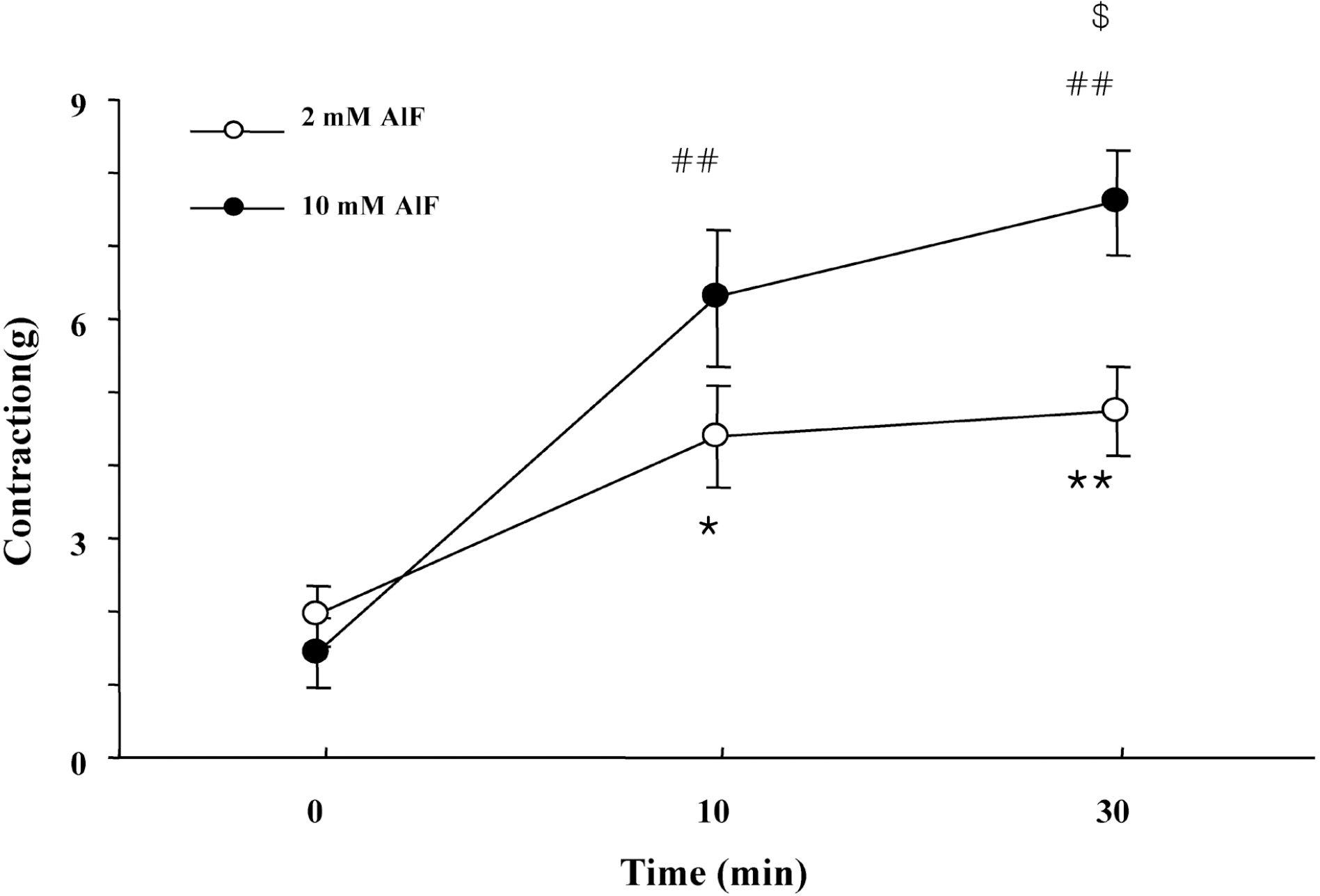
Fig. 4.
Effect of PTX, C3 exoenzyme and Y27632 on the on-contraction in the presence of L-NAME. (A) Muscle strips were pretreated with PTX or C3 exoenzyme for 3 hr, and then the contraction was measured. PTX significantly inhibited the contraction; in particular, co-treatment with PTX and C3 toxin additively inhibited the contraction. (B) Muscle strips were incubated with various concentration of Y27632 for 30 min before EFS, and then the contraction was measured Results are expressed as means±S.E. of 3 experiments. ∗p<0.05, ∗∗p<0.01, ∗∗∗p<0.001 compared with control of the on-contraction in the presence of L-NAME (ANOVA), ap<0.01 compared with PTX alone, bp<0.01 compared with C3 exoenzyme alone (ANOVA).
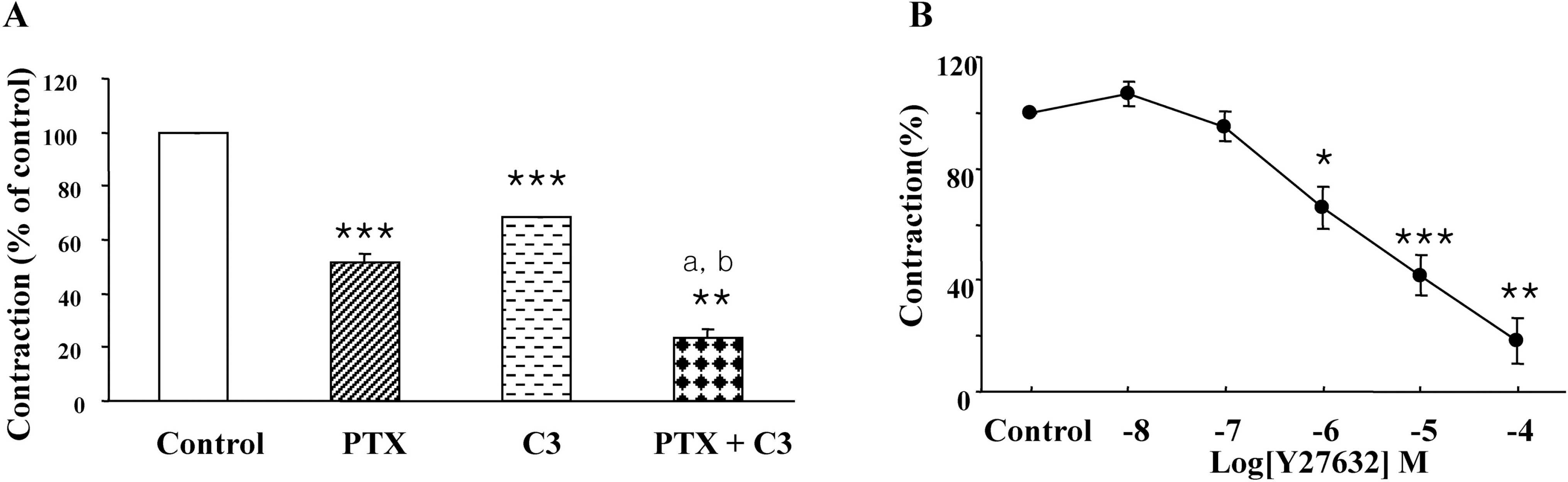
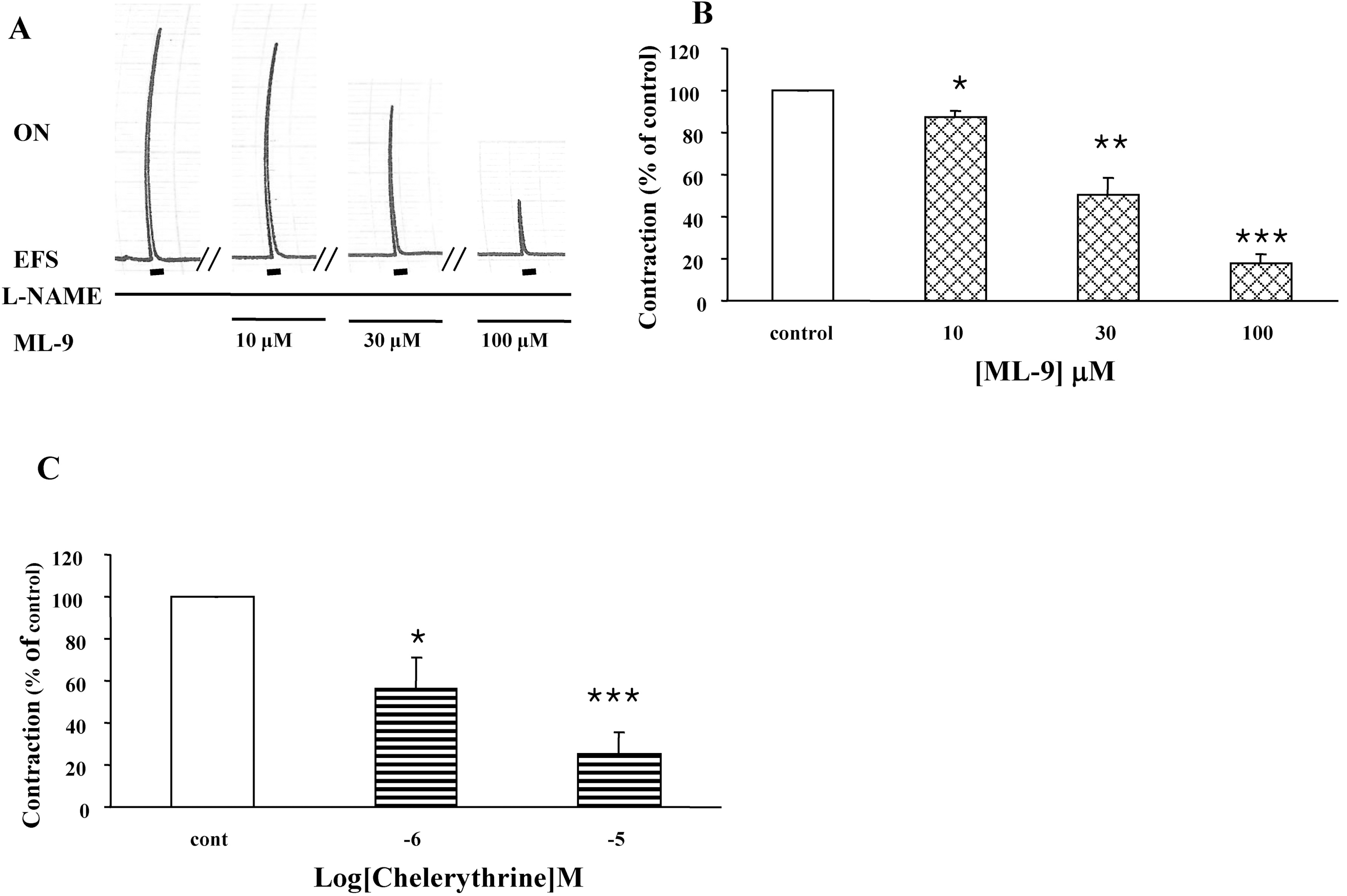
Fig. 5.
Effect of ML-9 or chelerythrine on the on-contraction in the presence of L-NAME. (A) Representative trace of the effect of various ML-9 on the on-contraction in the presence of L-NAME. (B) The changes in amplitudes of the contraction affected by ML-9 (10, 30, 100 μM) were expressed as % of control. (C) Chelerythrine (1, 10 μM) was added in the muscle bath before 20 min and then the contraction in response to EFS was measured. Results are expressed as means±S.E. of 3 experiments. ∗p<0.05, ∗∗p<0.01, ∗∗∗p<0.001 compared with control of on-contraction in the presence of L-NAME (ANOVA).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download