Abstract
Adenosine (Ado) is an important mediator of the endogenous defense against ischemia-induced injury in the heart. The action of Ado is mediated by activation of G protein-gated inwardly rectifying K+ (GIRK) channels. In turn, GIRK channels are inhibited by reducing phosphatidylinositol 4,5-bisphosphate (PIP2) through Gq protein-coupled receptors (GqPCRs). We previously found that GIRK channels activated by acetylcholine, a muscarinic M2 acetylcholine receptor agonist, are inhibited by GqPCRs in a receptor-specific manner. However, it is not known whether GIRK channels activated by Ado signaling are also regulated by GqPCRs. Presently, this was investigated in mouse atrial myocytes using the patch clamp technique. GIRK channels were activated by 100 μM Ado. When Ado was repetitively applied at intervals of 5 ∼ 6 min, the amplitude of second Ado-activated GIRK currents (IK(Ado)) was 88.3±3.7% of the first IK(Ado) in the control. Pretreatment of atrial myocytes with phenylephrine, endothelin-1, or bradykinin prior to a second application of Ado reduced the amplitude of the second IK(Ado) to 25.5±11.6%, 30.5±5.6%, and 96.0±2.7%, respectively. The potency of IK(Ado) inhibition by GqPCRs was different with that observed in acetylcholine-activated GIRK currents (IK(ACh)) (endothelin-1 > phenylephrine > bradykinin). IK(Ado) was almost completely inhibited by 500 μM of the PIP2 scavenger neomycin, suggesting low PIP2 affinity of IK(Ado). Taken together, these results suggest that the crosstalk between GqPCRs and the Ado-induced signaling pathway is receptor-specific. The differential change in PIP2 affinity of GIRK channels activated by Ado and ACh may underlie, at least in part, their differential responses to GqPCR agonists.
References
1. Babbitt DG, Virmani R, Forman MB. Intracoronary adenosine administered after reperfusion limits vascular injury after prolonged ischemia in the canine model. Circulation. 1989; 80:1388–1399.

2. Ely SW, Berne RM. Protective effects of adenosine in myocardial ischemia. Circulation. 1992; 85:893–904.

3. Gross GJ. ATP-sensitive potassium channels and myocardial preconditioning. Basic Res Cardiol. 1995; 90:85–88.

4. Li GC, Vasquez JA, Gallagher KP, Lucchesi BR. Myocardial protection with preconditioning. Circulation. 1990; 82:609–619.

5. Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986; 74:1124–1136.

6. Olafsson B, Forman MB, Puett DW, Pou A, Cates CU, Friesinger GC, Virmani R. Reduction of reperfusion injury in the canine preparation by intracoronary adenosine: importance of the endothelium and the no-reflow phenomenon. Circulation. 1987; 76:1135–1145.

7. Ely SW, Mentzer RM Jr, Lasley RD, Lee BK, Berne RM. Functional and metabolic evidence of enhanced myocardial tolerance to ischemia and reperfusion with adenosine. J Thorac Cardiovasc Surg. 1985; 90:549–556.

8. Reibel DK, Rovetto MJ. Myocardial ATP synthesis and mechanical function following oxygen deficiency. Am J Physiol. 1978; 234:H620–624.

9. Matherne GP, Linden J, Byford AM, Gauthier NS, Headrick JP. Transgenic A1 adenosine receptor overexpression increases myocardial resistance to ischemia. Proc Natl Acad Sci USA. 1997; 94:6541–6546.

10. Belardinelli L, Isenberg G. Isolated atrial myocytes: adenosine and acetylcholine increase potassium conductance. Am J Physiol. 1983; 244:H734–737.

11. Belardinelli L, Shryock JC, Song Y, Wang D, Srinivas M. Ionic basis of the electrophysiological actions of adenosine on cardiomyocytes. FASEB J. 1995; 9:359–365.

12. Bunemann M, Pott L. Down-regulation of A1 adenosine receptors coupled to muscarinic K+ current in cultured guineapig atrial myocytes. J Physiol. 1995; 482:81–92.
13. Kurachi Y, Nakajima T, Sugimoto T. On the mechanism of activation of muscarinic K+ channels by adenosine in isolated atrial cells: involvement of GTP-binding proteins. Pflugers Arch. 1986; 407:264–274.
14. Wellner-Kienitz MC, Bender K, Meyer T, Bunemann M, Pott L. Overexpressed A(1) adenosine receptors reduce activation of acetylcholine-sensitive K+ current by native muscarinic M(2) receptors in rat atrial myocytes. Circ Res. 2000; 86:643–648.
15. Krapivinsky G, Gordon EA, Wickman K, Velimirovic B, Krapivinsky L, Clapham DE. The G-protein-gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifying K+-channel proteins. Nature. 1995; 374:135–141.
16. Kubo Y, Reuveny E, Slesinger PA, Jan YN, Jan LY. Primary structure and functional expression of a rat G-protein-coupled muscarinic potassium channel. Nature. 1993; 364:802–806.

17. Sakmann B, Noma A, Trautwein W. Acetylcholine activation of single muscarinic K+ channels in isolated pacemaker cells of the mammalian heart. Nature. 1983; 303:250–253.
18. Kobayashi T, Ikeda K. G protein-activated inwardly rectifying potassium channels as potential therapeutic targets. Curr Pharm Des. 2006; 12:4513–4523.

19. Yamada M. The role of muscarinic K+ channels in the negative chronotropic effect of a muscarinic agonist. J Pharmacol Exp Ther. 2002; 300:681–687.
20. Ho IH, Murrell-Lagnado RD. Molecular mechanism for sodium-dependent activation of G protein-gated K+ channels. J Physiol. 1999; 520 Pt. 3:645–651.
21. Huang CL, Feng S, Hilgemann DW. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gbetagamma. Nature. 1998; 391:803–806.
22. Sui JL, Petit-Jacques J, Logothetis DE. Activation of the atrial KACh channel by the betagamma subunits of G proteins or intracellular Na+ ions depends on the presence of phosphatidylinositol phosphates. Proc Natl Acad Sci USA. 1998; 95:1307–1312.
23. Zhang H, He C, Yan X, Mirshahi T, Logothetis DE. Activation of inwardly rectifying K+ channels by distinct PtdIns(4,5)P2 interactions. Nat Cell Biol. 1999; 1:183–188.
24. Jenkinson S, Nahorski SR, Challiss RA. Disruption by lithium of phosphatidylinositol-4,5-bisphosphate supply and inositol-1,4,5-trisphosphate generation in Chinese hamster ovary cells expressing human recombinant m1 muscarinic receptors. Mol Pharmacol. 1994; 46:1138–1148.
25. Willars GB, Nahorski SR, Challiss RA. Differential regulation of muscarinic acetylcholine receptor-sensitive polyphosphoinositide pools and consequences for signaling in human neuroblastoma cells. J Biol Chem. 1998; 273:5037–5046.

26. Cho H, Nam GB, Lee SH, Earm YE, Ho WK. Phosphatidylinositol 4,5-bisphosphate is acting as a signal molecule in alpha(1)-adrenergic pathway via the modulation of acetylcholine-activated K+ channels in mouse atrial myocytes. J Biol Chem. 2001; 276:159–164.
27. Cho H, Lee D, Lee SH, Ho WK. Receptor-induced depletion of phosphatidylinositol 4,5-bisphosphate inhibits inwardly rectifying K+ channels in a receptor-specific manner. Proc Natl Acad Sci USA. 2005; 102:4643–4648.
28. Clerk A, Sugden PH. Regulation of phospholipases C and D in rat ventricular myocytes: stimulation by endothelin-1, bradykinin and phenylephrine. J Mol Cell Cardiol. 1997; 29:1593–1604.

29. Cho H, Hwang JY, Kim D, Shin HS, Kim Y, Earm YE, Ho WK. Acetylcholine-induced phosphatidylinositol 4,5-bisphosphate depletion does not cause short-term desensitization of G protein-gated inwardly rectifying K+ current in mouse atrial myocytes. J Biol Chem. 2002; 277:27742–27747.
30. Sohn JW, Lee D, Cho H, Lim W, Shin HS, Lee SH, Ho WK. Receptor-specific inhibition of GABAB-activated K+ currents by muscarinic and metabotropic glutamate receptors in immature rat hippocampus. J Physiol. 2007; 580:411–422.
31. Du X, Zhang H, Lopes C, Mirshahi T, Rohacs T, Logothetis DE. Characteristic interactions with phosphatidylinositol 4,5-bisphosphate determine regulation of kir channels by diverse modulators. J Biol Chem. 2004; 279:37271–37281.

32. Arbuzova A, Martushova K, Hangyas-Mihalyne G, Morris AJ, Ozaki S, Prestwich GD, McLaughlin S. Fluorescently labeled neomycin as a probe of phosphatidylinositol-4,5-bisphosphate in membranes. Biochim Biophys Acta. 2000; 1464:35–48.

33. Schulze D, Krauter T, Fritzenschaft H, Soom M, Baukrowitz T. Phosphatidylinositol 4,5-bisphosphate (PIP2) modulation of ATP and pH sensitivity in Kir channels. A tale of an active and a silent PIP2 site in the N terminus. J Biol Chem. 2003; 278:10500–10505.
34. Cho H, Youm JB, Earm YE, Ho WK. Inhibition of acetylcholine-activated K+ current by chelerythrine and bisindolylmaleimide I in atrial myocytes from mice. Eur J Pharmacol. 2001; 424:173–178.
35. Sohn JW, Lim A, Lee SH, Ho WK. Decrease in PIP(2) channel interactions is the final common mechanism involved in PKC-and arachidonic acid-mediated inhibitions of GABA(B)-activated K+ current. J Physiol. 2007; 582:1037–1046.
Fig. 1.
Differential activation of atrial GIRK currents by ACh and Ado. (A) Outward current activated by rapid exposure to Ado and ACh. The rapid deflections in this figure represent changes in membrane current due to ramp pulse from –120 to +60 mV, which were applied to obtain I-V curves of IK(Ado) and IK(ACh). Inset, the I-V curves for net IK(Ado) (black) IK(ACh) (gray). Data were calculated from data in left. The reversal potential was –70 mV. (B) Ado (100 μM) and ACh (100 μM) were applied for 2 min sequentially at a 6 min interval, as indicated by the horizontal lines above the trace. Inset, response to 100 μM Ado (black) and onset of response to 100 μM ACh (gray) on an expanded time scale. The response to 100 μM Ado was scaled up to match the amplitude of the response to 100 μM ACh. The arrow indicates the beginning of agonist exposure. (C) Summarized data for normalized peak amplitude of IK(Ado) to that of IK(ACh).
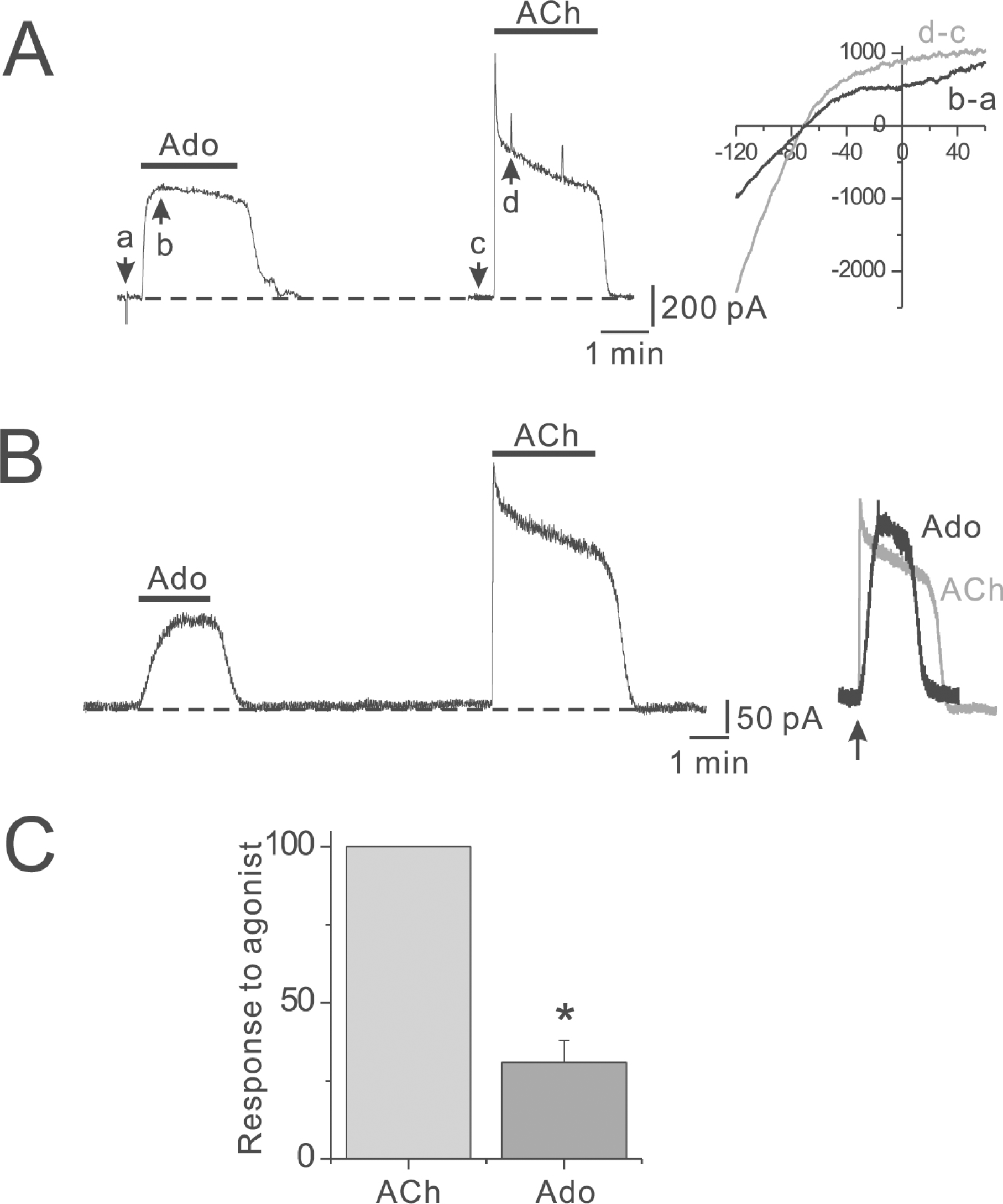
Fig. 2.
Differential regulation of IK(Ado) by different GqPCRs. (A) Ado (100 μM) was applied for 2 min twice at a 6 min interval, as indicated by the horizontal lines above the trace. (B∼D) Phenylephrine (PE, 100 μM), endothelin-1 (ET-1, 30 nM), or bradykinin (BK, 10 μM) was applied prior to the application of Ado as indicated by the horizontal lines above the trace. (E) Summarized data for potency of IK(Ado) inhibition. The numbers in parentheses indicate numbers of cells. ∗p<0.01. Error bars indicate S.E.M.
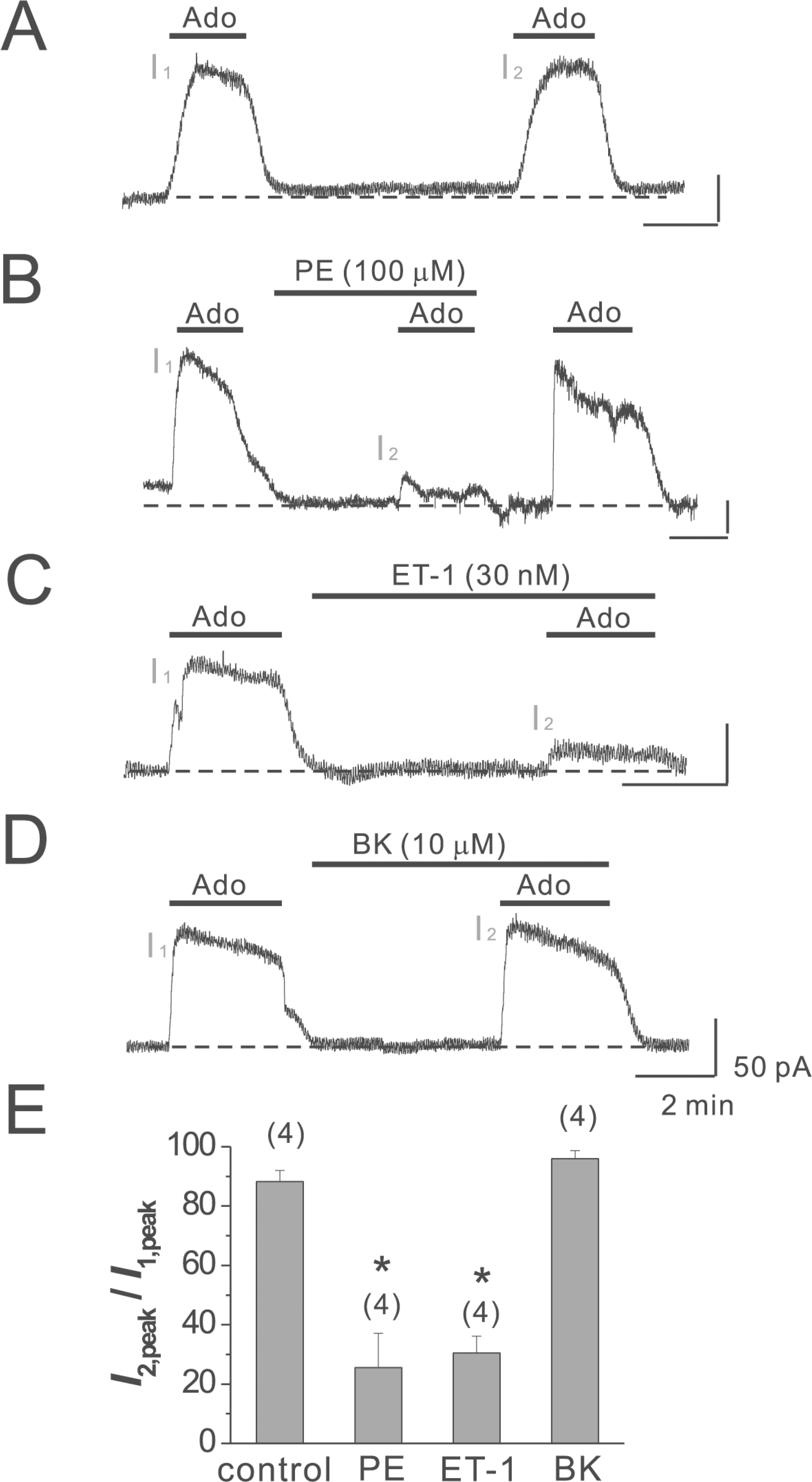
Fig. 3.
PDBu has little effect on IK(Ado) in mouse atrial myocytes. (A) PDBu (100 nM), a PKC activator, was applied as a pretreatment before the activation of I2. (B) the I2, peak in the absence and presence of PDBu was 88.3±3.7% (n=4) and 97.5±0.5% (n=5) of I1,peak, respectively. The numbers in parentheses indicate numbers of cells. Error bars indicate S.E.M.
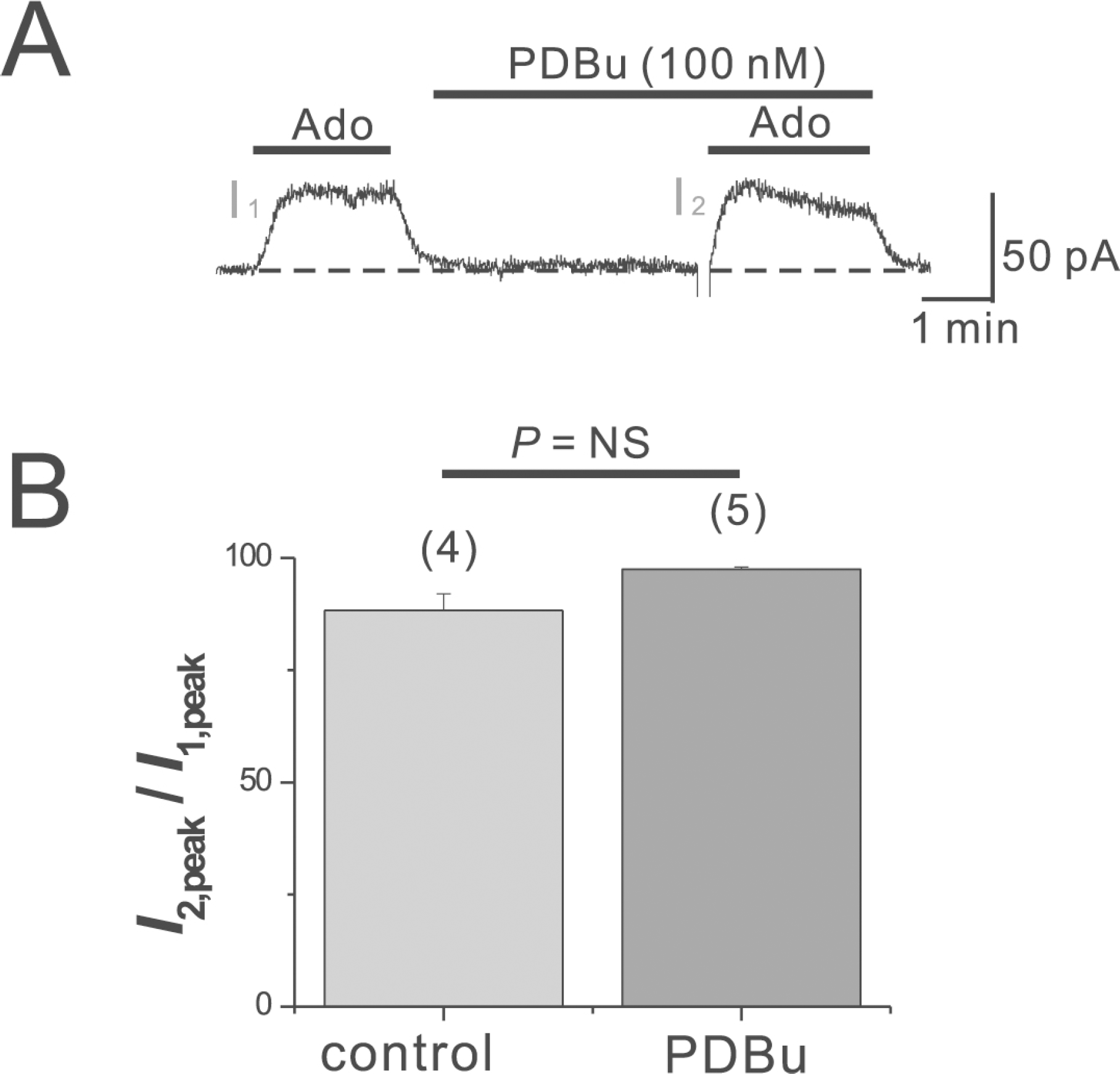
Fig. 4.
Neomycin inhibition of IK(Ado). (A, B) Neomycin at concentrations of 500 μM (A) or 100 μM (B) was applied prior to the application of Ado as indicated by the horizontal lines above the trace. (C) Summarized data for the neomycin inhibition of IK(Ado). The numbers in parentheses indicate numbers of cells. ∗p<0.01. Error bars indicate S.E.M.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download