Abstract
In this study, we evaluated the role of apurinic/apyrimidinic endonuclease1/redox factor-1 (Ref-1) on the tumor necrosis factor-α (TNF-α) induced cyclooxygenase-2 (COX-2) expression using A549 lung adenocarcinoma cells. TNF-α induced the expression of COX-2 in A549 cells, but did not induce BEAS-2B expression. The expression of COX-2 in A549 cells was TNF-α dose-dependent (5∼100 ng/ml). TNF-α-stimulated A549 cells evidenced increased Ref-1 expression in a dose-dependent manner. The adenoviral transfection of cells with AdRef-1 inhibited TNF-α-induced COX-2 expression relative to that seen in the control cells (Adβgal). Pretreatment with 10 μM of SB203580 suppressed TNF-α-induced COX-2 expression, thereby suggesting that p38 MAPK might be involved in COX-2 expression in A549 cells. The phosphorylation of p38 MAPK was increased significantly after 5 minutes of treatment with TNF-α, reaching a maximum level at 10 min which persisted for up to 60 min. However, p38MAPK phosphorylation was markedly suppressed in the Ref-1-overexpressed A549 cells. Taken together, our results appear to indicate that Ref-1 negatively regulates COX-2 expression in response to cytokine stimulation via the inhibition of p38 MAPK phosphorylation. In the lung cancer cell lines, Ref-1 may be involved as an important negative regulator of inflammatory gene expression.
References
1. Fosslien E. Molecular pathology of cyclooxygenase-2 in neoplasia. Ann Clin Lab Sci. 2000; 30:3–21.
2. Soslow RA, Dannenberg AJ, Rush D, Woerner BM, Khan KN, Masferrer J, Koki AT. COX-2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer. 2000; 89:2637–2645.

3. Yip-Schneider MT, Barnard DS, Billings SD, Cheng L, Heilman DK, Lin A, Marshall SJ, Crowell PL, Marshall MS, Sweeney CJ. Cyclooxygenase-2 expression in human pancreatic adenocarcinomas. Carcinogenesis. 2000; 21:139–146.

4. Shamma A, Yamamoto H, Doki Y, Okami J, Kondo M, Fujiwara Y, Yano M, Inoue M, Matsuura N, Shiozaki H, Monden M. Upregulation of cyclooxygenase-2 in squamous carcinogenesis of the esophagus. Clin Cancer Res. 2000; 6:1229–1238.
5. Chan G, Boyle JO, Yang EK, Zhang F, Sacks PG, Shah JP, Edelstein D, Soslow RA, Koki AT, Woerner BM, Masferrer JL, Dannenberg AJ. Cyclooxygenase-2 expression is up-regulated in squamous cell carcinoma of the head and neck. Cancer Res. 1999; 59:991–994.
6. Hida T, Yatabe Y, Achiwa H, Muramatsu H, Kozaki K, Nakamura S, Ogawa M, Mitsudomi T, Sugiura T, Takahashi T. Increased expression of cyclooxygenase 2 occurs frequently in human lung cancers, specifically in adenocarcinomas. Cancer Res. 1998; 58:3761–3764.
7. Hida T, Kozaki K, Muramatsu H, Masuda A, Shimizu S, Mitsudomi T, Sugiura T, Ogawa M, Takahashi T. Cyclooxygenase-2 inhibitor induces apoptosis and enhances cytotoxicity of various anticancer agents in non-small cell lung cancer cell lines. Clin Cancer Res. 2000; 6:2006–2011.
8. Huang M, Stolina M, Sharma S, Mao JT, Zhu L, Miller PW, Wollman J, Herschman H, Dubinett SM. Non-small cell lung cancer cyclooxygenase-2-dependent regulation of cytokine balance in lymphocytes and macrophages: up-regulation of interleukin 10 and down-regulation of interleukin 12 production. Cancer Res. 1998; 58:1208–1216.
9. Dohadwala M, Luo J, Zhu L, Lin Y, Dougherty GJ, Sharma S, Huang M, Pold M, Batra RK, Dubinett SM. Non-small cell lung cancer cyclooxygenase-2-dependent invasion is mediated by CD44. J Biol Chem. 2001; 276:20809–20812.

10. Altorki NK, Keresztes RS, Port JL, Libby DM, Korst RJ, Flieder DB, Ferrara CA, Yankelevitz DF, Subbaramaiah K, Pasmantier MW, Dannenberg AJ. Celecoxib, a selective cyclo-oxygenase-2 inhibitor, enhances the response to preoperative paclitaxel and carboplatin in early-stage non-small-cell lung cancer. J Clin Oncol. 2003; 21:2645–2650.

11. Castelao JE, Bart RD 3rd, DiPerna CA, Sievers EM, Bremner RM. Lung cancer and cyclooxygenase-2. Ann Thorac Surg. 2003; 76:1327–1335.

12. Backhus LM, Sievers E, Lin GY, Castanos R, Bart RD, Starnes VA, Bremner RM. Perioperative cyclooxygenase 2 inhibition to reduce tumor cell adhesion and metastatic potential of circulating tumor cells in non-small cell lung cancer. J Thorac Cardiovasc Surg. 2006; 132:297–303.

14. Tomicic M, Eschbach E, Kaina B. Expression of yeast but not human apurinic/apyrimidinic endonuclease renders Chinese hamster cells more resistant to DNA damaging agents. Mutat Res. 1997; 383:155–165.

15. Kim CS, Son SJ, Kim EK, Kim SN, Yoo DG, Kim HS, Ryoo SW, Lee SD, Irani K, Jeon BH. Apurinic/apyrimidinic endonuclease1/redox factor-1 inhibits monocyte adhesion in endothelial cells. Cardiovasc Res. 2006; 69:520–526.

16. Yoo DG, Song YJ, Cho EJ, Lee SK, Park JB, Yu JH, Lim SP, Kim JM, Jeon BH. Alteration of APE1/ref-1 expression in non-small cell lung cancer: the implications of impaired extracellular superoxide dismutase and catalase antioxidant systems. Lung Cancer. 2008; 60:277–284.

17. Tell G, Damante G, Caldwell D, Kelley MR. The intracellular localization of APE1/Ref-1: more than a passive phenomenon? Antioxid Redox Signal. 2005; 7:367–384.

18. Pines A, Perrone L, Bivi N, Romanello M, Damante G, Gulisano M, Kelley MR, Quadrifoglio F, Tell G. Activation of APE1/Ref-1 is dependent on reactive oxygen species generated after purinergic receptor stimulation by ATP. Nucleic Acids Res. 2005; 33:4379–4394.

19. Lee HM, Yuk JM, Shin DM, Yang CS, Kim KK, Choi DK, Liang ZL, Kim JM, Jeon BH, Kim CD, Lee JH, Jo EK. Apurinic/apyrimidinic endonuclease 1 is a key modulator of keratinocyte inflammatory responses. J Immunol. 2009; 183:6839–6848.

20. Reddel RR, Ke Y, Gerwin BI, McMenamin MG, Lechner JF, Su RT, Brash DE, Park JB, Rhim JS, Harris CC. Transformation of human bronchial epithelial cells by infection with SV40 or adenovirus-12 SV40 hybrid virus, or transfection via strontium phosphate coprecipitation with a plasmid containing SV40 early region genes. Cancer Res. 1988; 48:1904–1909.
21. Bhakat KK, Mantha AK, Mitra S. Transcriptional regulatory functions of mammalian AP-endonuclease (APE1/Ref-1), an essential multifunctional protein. Antioxid Redox Signal. 2009; 11:621–638.

22. Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001; 11:372–377.

23. Mitsudomi T, Viallet J, Mulshine JL, Linnoila RI, Minna JD, Gazdar AF. Mutations of ras genes distinguish a subset of non-small-cell lung cancer cell lines from small-cell lung cancer cell lines. Oncogene. 1991; 6:1353–1362.
24. Subbaramaiah K, Telang N, Ramonetti JT, Araki R, DeVito B, Weksler BB, Dannenberg AJ. Transcription of cyclooxygenase-2 is enhanced in transformed mammary epithelial cells. Cancer Res. 1996; 56:4424–4429.
25. Hosomi Y, Yokose T, Hirose Y, Nakajima R, Nagai K, Nishiwaki Y, Ochiai A. Increased cyclooxygenase 2 (COX-2) expression occurs frequently in precursor lesions of human adenocarcinoma of the lung. Lung Cancer. 2000; 30:73–81.

26. Wolff H, Saukkonen K, Anttila S, Karjalainen A, Vainio H, Ristimaki A. Expression of cyclooxygenase-2 in human lung carcinoma. Cancer Res. 1998; 58:4997–5001.
27. Fang HY, Lin TS, Lin JP, Wu YC, Chow KC, Wang LS. Cyclooxygenase-2 in human non-small cell lung cancer. Eur J Surg Oncol. 2003; 29:171–177.

28. Ermert L, Dierkes C, Ermert M. Immunohistochemical expression of cyclooxygenase isoenzymes and downstream enzymes in human lung tumors. Clin Cancer Res. 2003; 9:1604–1610.
29. Han S, Roman J. COX-2 inhibitors suppress lung cancer cell growth by inducing p21 via COX-2 independent signals. Lung Cancer. 2006; 51:283–296.

30. Dalaveris E, Kerenidi T, Katsabeki-Katsafli A, Kiropoulos T, Tanou K, Gourgoulianis KI, Kostikas K. VEGF, TNF-alpha and 8-isoprostane levels in exhaled breath condensate and serum of patients with lung cancer. Lung Cancer. 2009; 64:219–225.
31. Derin D, Soydinc HO, Guney N, Tas F, Camlica H, Duranyildiz D, Yasasever V, Topuz E. Serum levels of apoptosis biomarkers, survivin and TNF-alpha in nonsmall cell lung cancer. Lung Cancer. 2008; 59:240–245.

33. Lin CH, Kuan IH, Wang CH, Lee HM, Lee WS, Sheu JR, Hsiao G, Wu CH, Kuo HP. Lipoteichoic acid-induced cyclooxygenase-2 expression requires activations of p44/42 and p38 mitogen-activated protein kinase signal pathways. Eur J Pharmacol. 2002; 450:1–9.

34. N'Guessan PD, Etouem MO, Schmeck B, Hocke AC, Scharf S, Vardarova K, Opitz B, Flieger A, Suttorp N, Hippenstiel S. Legionella pneumophila-induced PKCalpha-, MAPK-, and NF-kappaB-dependent COX-2 expression in human lung epithelium. Am J Physiol Lung Cell Mol Physiol. 2007; 292:L267–277.
35. Wei J, Yan W, Li X, Chang WC, Tai HH. Activation of thromboxane receptor alpha induces expression of cyclooxygenase-2 through multiple signaling pathways in A549 human lung adenocarcinoma cells. Biochem Pharmacol. 2007; 74:787–800.
36. Angkeow P, Deshpande SS, Qi B, Liu YX, Park YC, Jeon BH, Ozaki M, Irani K. Redox factor-1: an extra-nuclear role in the regulation of endothelial oxidative stress and apoptosis. Cell Death Differ. 2002; 9:717–725.

37. Lee HM, Jeon BH, Won KJ, Lee CK, Park TK, Choi WS, Bae YM, Kim HS, Lee SK, Park SH, Irani K, Kim B. Gene transfer of redox factor-1 inhibits neointimal formation: involvement of platelet-derived growth factor-beta receptor signaling via the inhibition of the reactive oxygen species-mediated Syk pathway. Circ Res. 2009; 104:219–227.
Fig. 1.
Tumor necrosis factor-α (TNF-α) induced cyclooxygenase-2 (COX-2) expression in the A549 cells. (A) The effect of TNF-α on COX-2 expression in BEAS-2B and A549 cells. COX-2 expression was analyzed via Western blotting 24 hr after exposure to TNF-α (30∼100 ng/ml). (B) COX-2 expression in A549 cells evidenced dose-dependency to TNF-α (5∼100 ng/ml). (C) The time-dependent change of COX-2 expression in the response to TNF-α (30 ng/ml) in A549 cells.
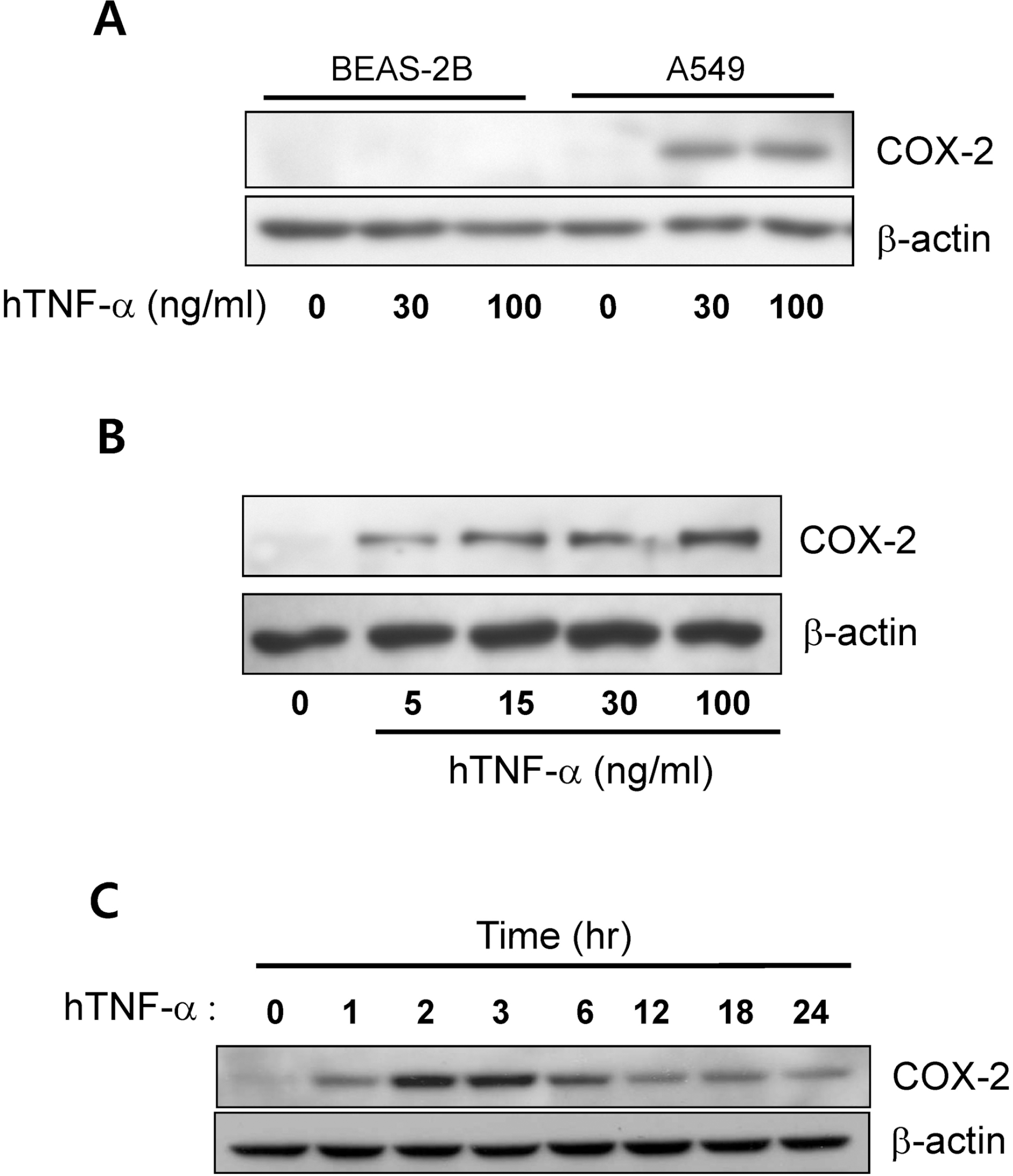
Fig. 2.
Tumor necrosis factor-α (TNF-α) induced Ref-1 expression in the A549 cells. (A) Effect of TNF-α on the Ref-1 expression in the A549 cells. Ref-1 expression was analyzed via Western blotting 24 hr after exposure to TNF-α (30∼100 ng/ml). β-actin was used as a loading control. (B) Densitometry analysis for Ref-1 expression. Each bar shows the mean±S.E. (n=4), ∗p<0.05, ∗∗p<0.01.
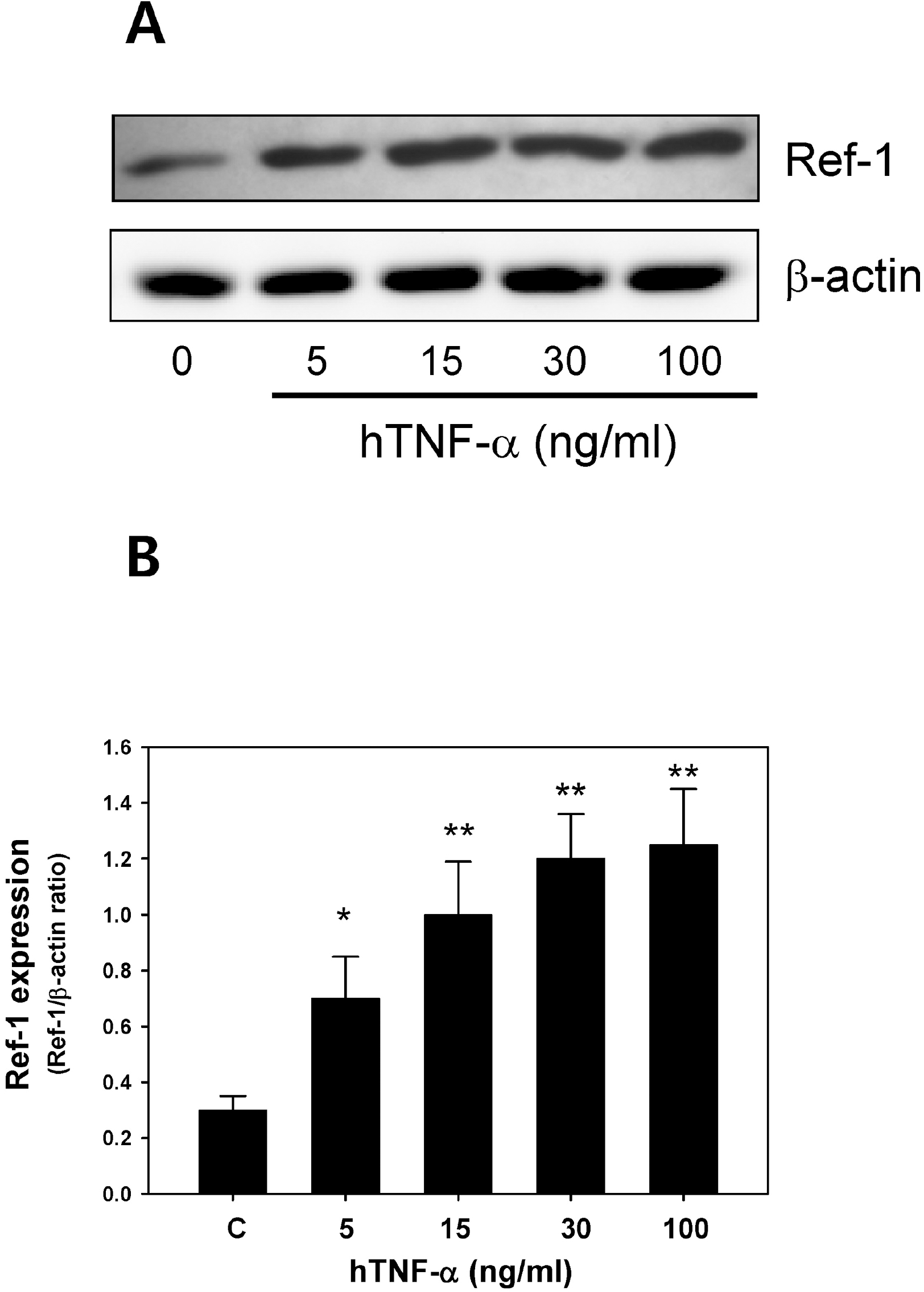
Fig. 3.
Ref-1 inhibited COX-2 expression in the A549 cells. (A) Western blots for COX-2 and Ref-1. After the cells were treated with AdRef-1 (0∼500 MOI), the cells were stimulated with TNF-α (30 ng/ml) for 18 hr. Western blotting was conducted using anti-COX-2 and anti-Ref-1 antibodies. The total adenovirus titer was balanced with Adβgal (500 MOI). (B) Densitometry analysis for COX-2 expression. Each bar showed the mean±S.E. (n=3), ∗p<0.05, ∗∗p<0.01.
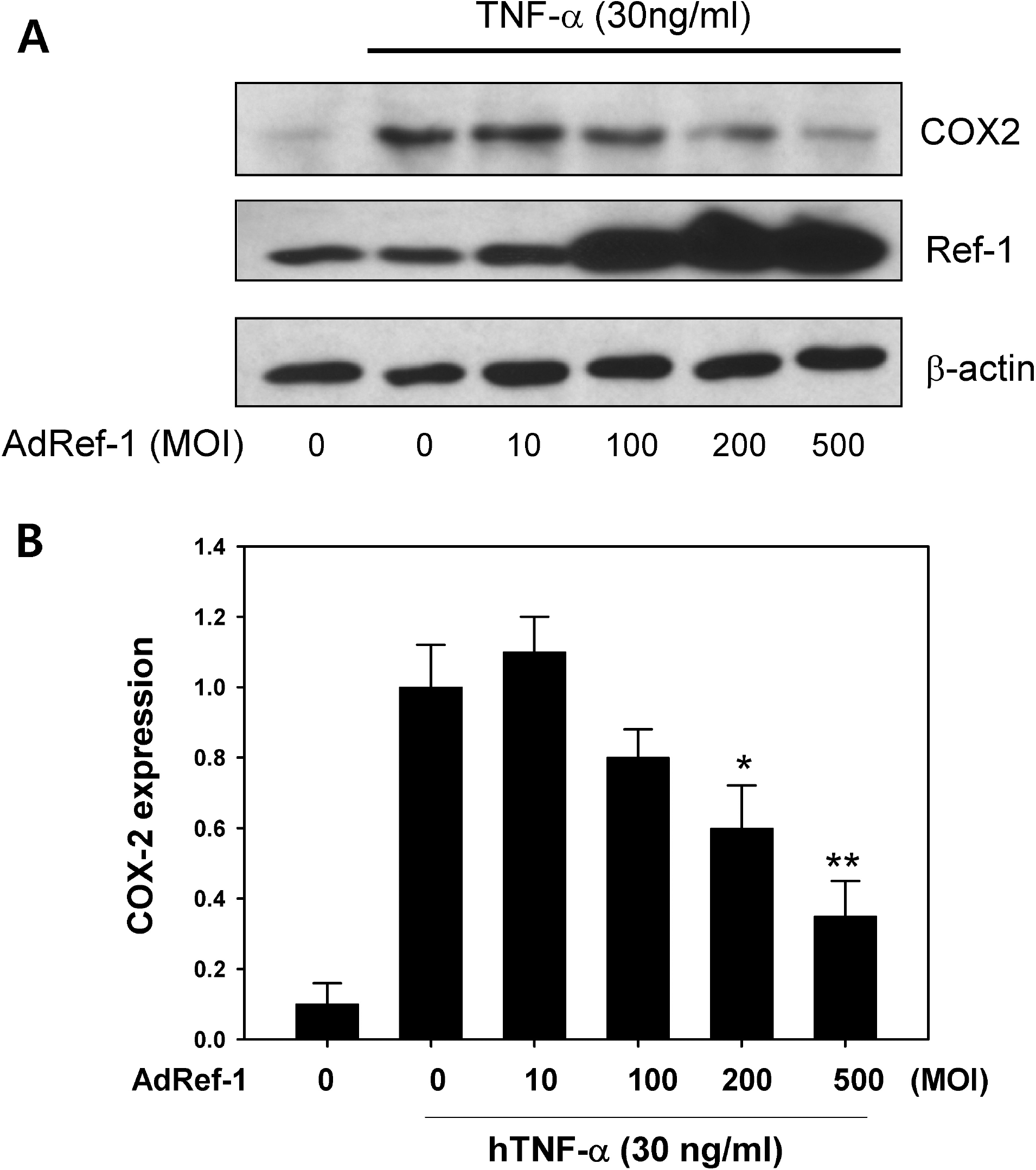
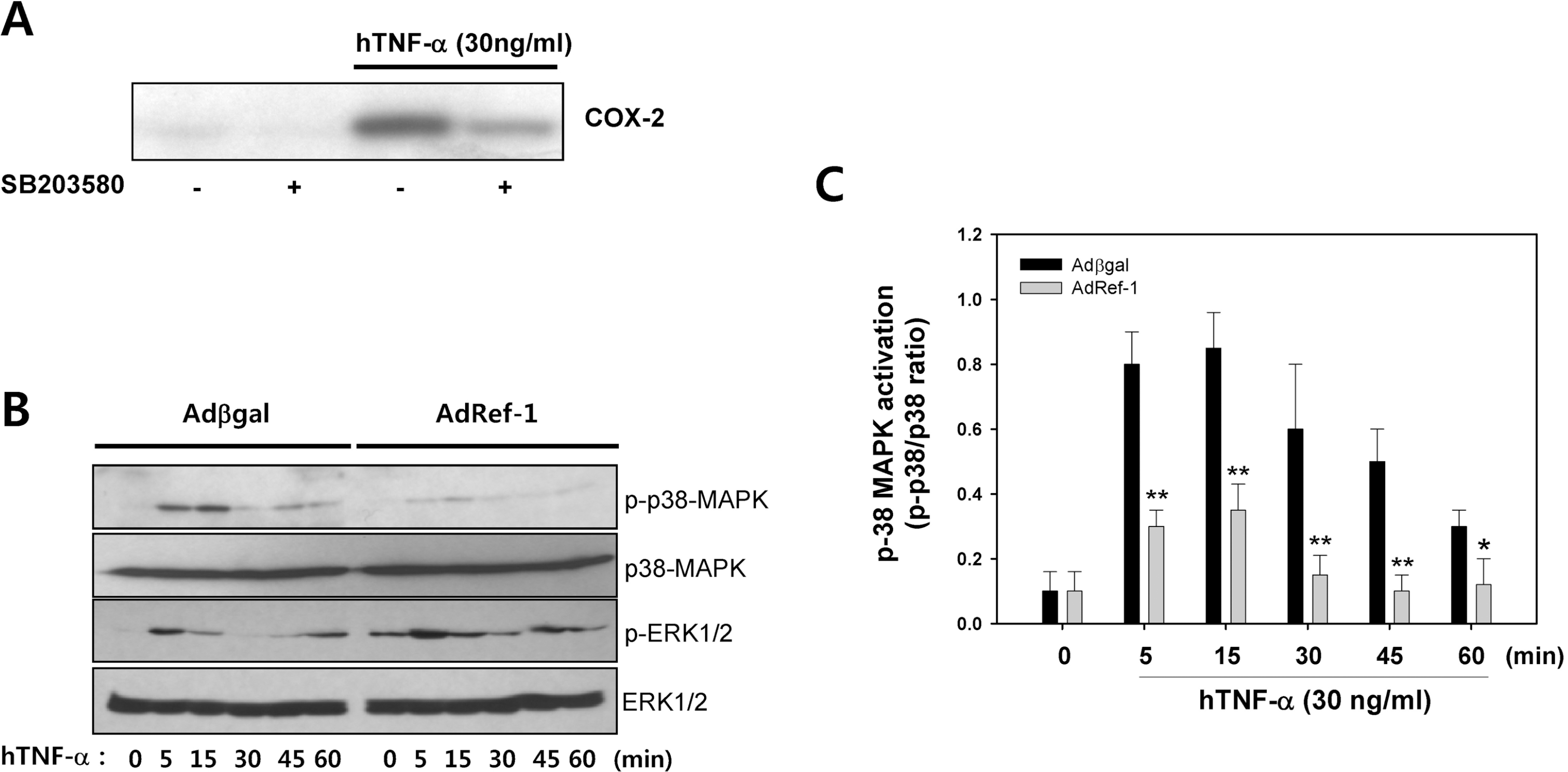
Fig. 4.
Ref-1 inhibited p38 MAPK activation in the A549 cells. (A) TNF-α induced COX-2 expression was inhibited by SB203580 (10 μM), an inhibitor of p38 MAPK. (B) Time-dependent change of p38 MAPK activation (p-p38 MAPK) and ERK activation (p-ERK) in the response of TNF-α in the Adβgal or AdRef-1-transfected A549 cells. (C) Densitometry analysis for phospho-p38 MAPK expression. Each bar showed the mean±S.E. (n=4), ∗∗p<0.01.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download