Abstract
We investigated the effects of a hot-water extract of Artemisia iwayomogi, a plant belonging to family Compositae, on cardiac ventricular delayed rectifier K+ current (IK) using the patch clamp technique. The carbohydrate fraction AIP1 dose-dependently increased the heart rate with an apparent EC50 value of 56.1±5.5 μg/ml. Application of AIP1 reduced the action potential duration (APD) in concentration-dependent fashion by activating IK without significantly altering the resting membrane potential (IC50 value of APD50: 54.80±2.24, IC50 value of APD90: 57.45±3.47 μg/ml). Based on the results, all experiments were performed with 50 μg/ml of AIP1. Pre-treatment with the rapidly activating delayed rectifier K+ current (IKr) inhibitor, E-4031 prolonged APD. However, additional application of AIP1 did not reduce APD. The inhibition of slowly activating delayed rectifier K+ current (IKs) by chromanol 293B did not change the effect of AIP1. AIP1 did not significantly affect coronary arterial tone or ion channels, even at the highest concentration of AIP1. In summary, AIP1 reduces APD by activating IKr but not IKs. These results suggest that the natural product AIP1 may provide an adjunctive therapy of long QT syndrome.
References
1. Sanguinetti MC, Jurkiewicz NK. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. J Gen Physiol. 1990; 96:195–215.
2. Zeng J, Laurita KR, Rosenbaum DS, Rudy Y. Two components of the delayed rectifier K+ current in ventricular myocytes of the guinea pig type. Theoretical formation and their role in repolarization. Circ Res. 1995; 77:140–152.
3. Heath BM, Terrar DA. Separation of the components of the delayed rectifier potassium current using selective blockers of IKr and IKs in guinea-pig isolated ventricular myocytes. Exp Physiol. 1996a; 81:587–603.
4. Heath BM, Terrar DA. The deactivation kinetics of the delayed rectifier components IKr and IKs in guinea-pig isolated ventricular myocytes. Exp Physiol. 1996b; 81:605–621.
5. Chiang CE, Roden DM. The long QT syndromes: genetic basis and clinical implications. J Am Coll Cardiol. 2000; 36:1–12.

6. Salata JJ, Jurkiewicz NK, Wang J, Evans BE, Orme HT, Sanguinetti MC. A novel benzodiazepine that activates cardiac slow delayed rectifier K+ currents. Mol Pharmacol. 1998; 53:220–230.
7. Keating MT, Sanguinetti MC. Molecular and cellular mechanisms of cardiac arrhythmias. Cell. 2001; 104:569–580.

8. Lee KR, Zee OP, Kwak JH, Kim YS, Park HK, Koo KA, Youn HJ. The polysaccharide fractions of Artemisia species. Korean J Pharmacogn. 1993; 24:289–295.
9. Park EJ, Nan JX, Kim JY, Kang HC, Choi JH, Lee SJ, Lee BH, Lee JH, Kim YC, Sohn DW. The ethanol-soluble part of a hot-water extract from Artemisia iwayomogi inhibits liver fibrosis induced by carbon tetrachloride in rats. J Pharm Pharmacol. 2000; 52:875–881.
10. Koo KA, Kwak JW, Lee KR, Zee OP, Woo ER, Park HK, Youn HJ. Antitumor and immuno-modulating activities of the polysaccharide fractions from Artemisia selengenesis and Artemisia iwayomogi. Arch Pharm Res. 1994; 17:371–374.
11. Hwang JS, Chung HK, Bae EK, Lee AY, Ji HJ, Park DW, Jung HJ, Cho CW, Choi HJ, Lee DS, Lee KR, Youn HJ. The polysaccharide fraction AIP1 from Artemisia iwayomogi suppresses apoptotic death of the mouse spleen cells in culture. Arch Pharm Res. 2003; 26:294–300.
12. Hwang JS, Ji HJ, Koo KA, Lee NH, Yeo HK, Cheong SW, Park JH, Oh GS, Yoon CS, Youn HJ. AIP1, a water-soluble fraction from Artemisia iwayomogi, suppresses thymocyte apoptosis in Vitro and down-regulates the expression of Fas gene. Biol Pharm Bull. 2005; 28:921–924.
13. Ji HJ, Yeo HK, Lee NH, Hwang JS, Koo KA, Cheong SW, Park JH, Oh GS, Yoon CS, Youn HJ. A carbohydrate graction, AIP1, from Artemisia iwayomogi down-regulated Fas gene expression and suppresses apoptotic death of the thymocytes induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Biotechnol Lett. 2005; 27:253–257.
14. Kim NR, Youm JB, Joo H, Kim EY, Han J. Protein kinase C activates ATP-sensitive potassium channels in rabbit ventricular myocytes. Korean J Physiol Pharmacol. 2005; 9:187–193.
15. Park WS, Ko JH, Kim NR, Son YK, Kang SH, Warda M, Jung ID, Park YM, Han J. Increased inhibition of inward rectifier K+ channels by angiotensin II in small-diameter coronary artery of isoproterenol-induced hypertrophied model. Arterioscler Thromb Vasc Biol. 2007; 27:1768–1775.
16. Lengyel C, Iost N, Virag L, Varro A, Lathrop DA, Papp JG. Pharmacological block of the slow component of the outward delayed rectifier current (IKs) fails to lengthen rabbit ventricular muscle QTc and action potential duration. Br J Pharmacol. 2001; 132:101–110.
17. Volders PG, Stengl M, van Opstal JM, Gerlach U, Spätjens RL, Beekman JD, Sipido KR, Vos MA. Probing the contribution of IKs to canine ventricular repolarization: Key role for beta-adrenergic receptor stimulation. Circulation. 2003; 107:2753–2760.
18. Guerard NC, Traebert M, Suter W, Dumotier BM. Selective block of IKs plays a significant role in MAP triangulation induced by IKr block in isolated rabbit heart. J Pharmacol Toxicol Methods. 2008; 58:32–40.
19. Chung HK, Bae EK, Ji HJ, Hwang JS, Park DW, Kim EJ, Jung HJ, Choi HJ, Lee DS, Youn HJ. An oligosaccharide fraction from Korean mugwort herb suppresses death of the mouse thymocytes in culture by down-regulating the Fas death receptor gene. Biotechnol Lett. 2003; 25:1549–1553.
20. Kim SH, Choi CH, Kim SY, Eun JS, Shin TY. Anti-allergic effects of Artemisia iwayomogi on mast cell-mediated allegy model. Exp Biol Med. 2005; 230:82–88.
21. Lee JA, Sung HN, Jeon CH, Gill BC, Oh GS, Youn HJ, Park JH. A carbohydrate fraction, AIP1 from Artemisia iwayomogi suppresses pulmonary eosinophilia and Th2-type cytokine production in an ovalbumin-induced allergic asthma. Down-regulation of TNF-α expression in the lung. Int Immunopharmacol. 2008; 8:117–125.
22. Ahn H, Kim JY, Lee HJ, Kim YK, Ryu JH. Inhibitors of inducible nitric oxide synthase expression from Artemisia iwayomogi. Arch Pharm Res. 2003; 26:301–305.
23. Kim AR, Zou YN, Park TH, Shim KH, Kim MS, Kim ND, Kim JD, Bae SJ, Choi JS, Chung HY. Active components from Artemisia iwayomogi displaying ONOO-scavenging activity. Phytother Res. 2004; 18:1–7.
24. Takumi T, Ohkubo H, Nakanishi S. Cloning of a membrane protein that induced a slow voltage-gated potassium current. Science. 1988; 242:1042–1045.
25. Folaander K, Smith JS, Antanavage J, Bennett C, Stein RB, Swanson R. Cloning and expression of the delayed rectifier IKs channel from neonatal rat heart and diethylstilbestrol-primed rat uterus. Proc Natl Acad Sci USA. 1990; 87:2975–2979.
26. Freeman LS, Kass RS. Expression of a minimal K+ channel protein in mammalian cells and immunolocalization in guinea pig heart. Circ Res. 1993; 73:968–973.
27. Sanguinetti MC, Jiang C, Curran ME, Keating MT. A mechanistic link between and inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995; 81:299–307.
28. Sanguinetti MC, Curran ME, Zou A, Shen J, Spector PS, Atkinson DL, Keating MT. Coassembly of KvLQT1 and mink (IKs) proteins to form cardiac IKs potassium channel. Nature. 1996; 384:80–83.
29. Wang L, Feng Z, Kondo CS, Sheldon RS, Duff HJ. Developmental changes in the delayed rectifier K+ channels in mouse heart. Circ Res. 1996; 79:79–85.
30. Hansen RS, Olesen SP, Ronn LC, Grunnet M. In vivo effects of the IKr agonist NS 3623 on cardiac electrophysiology of the guinea pig. J Cardiovasc Pharmacol. 2008; 52:35–41.
31. Heath BM, Terrar DA. Protein kinase C enhances the rapidly activating delayed rectifier potassium current, IKr, through a reduction in C-type inactivation in guinea-pig ventricular myocytes. J Physiol. 2000; 522:391–402.
32. Yuan XJ, Wang J, Juhaszova M, Golovina VA, Rubin LJ. Molecular basis and function of voltage-gated K+ channels in pulmonary arterial smooth muscle cells. Am J Physiol. 1998; 274:L621–635.
33. Platoshyn O, Yu Y, Golovina VA, McDaniel SS, Krick S, Li L, Wang JY, Rubin LJ, Yuan JX. Chronic hypoxia decrease K(V) channel expression and function in pulmonary artery myocytes. Am J Physiol. 2001; 280:L801–812.
Fig. 1.
Effect of AIP1 on the heart rate and action potentials of ventricular myocytes. (A) Does-dependent effect of AIP1 on the heart rate (All n=4 hearts). (B) Dose-dependent effect of AIP1 on APD. Superimposed traces of APs obtained using the patch-clamp technique under control conditions and in various concentrations of AIP1 with an apparent IC50 value of APD50 and APD90 (n=4 cells). (C) Representative traces of APs under control conditions in the presence of 50 μg/ml AIP1. (D, E) Summary of the APD50 and APD90 as shown in (C). APD50 and APD90 mean the time required for repolarization to 50% and to 90% of basal membrane potential, respectively. ∗p<0.05 (n=6 cells).
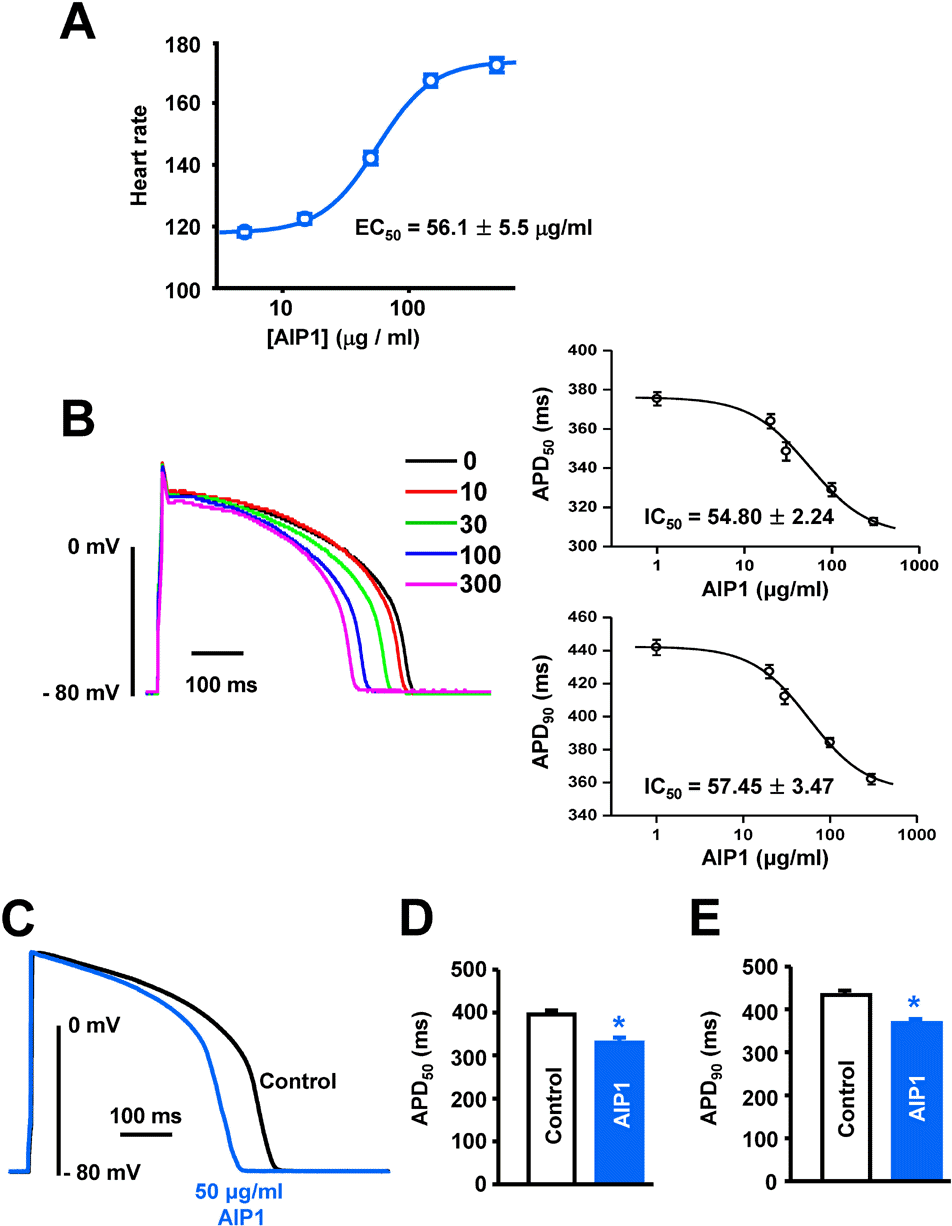
Fig. 2.
AIP1 increased IK in ventricular cardiomyocyte. (A, B) Whole-cell current of ventricular cardiomyocytes under control conditions (A) and in the presence of 50 μg/ml AIP1 (B). Panel (a) was original recording traces and panel (b) was magnified traces of the panel (a). (C) Summary of whole-cell I-V relationship under control conditions (❍) and in the presence of 50 μg/ml AIP1 (). (D) Statistical summary of IK at +40 mV. ∗p<0.05 (n=5 cells).
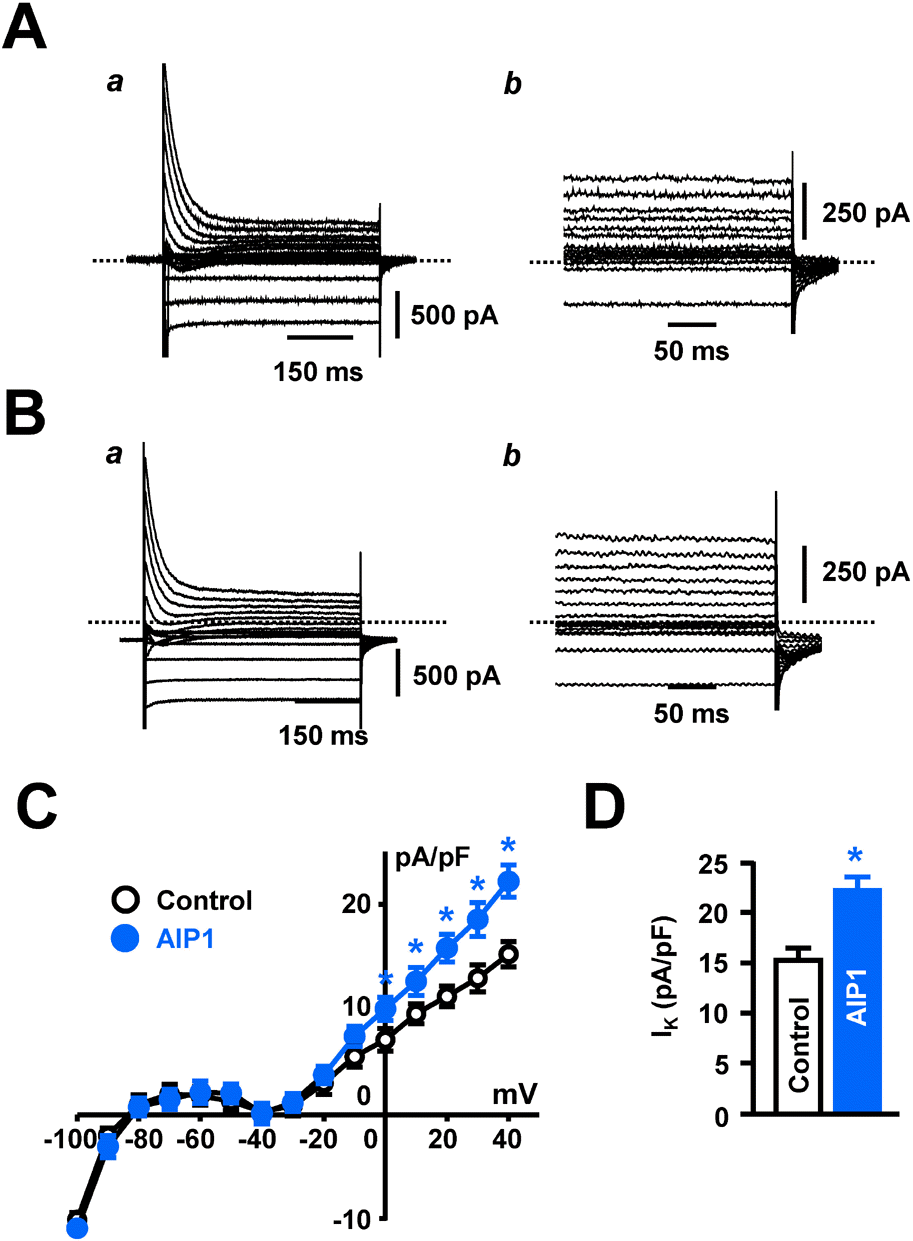
Fig. 3.
The effect of AIP1 on ICa in ventricular cardiomyocyte. (A) ICa in ventricular cardiomyocytes under control conditions and in the presence of 50 μg/ml AIP1. ICa was confirmed using the ICa inhibitor, nifedipine (10 μM). (B) Statistical summary of ICa at 0 mV. NS, not significant (Control vs AIP1, n=4 cells). ∗p<0.05 (Control vs nifedipine, n=4 cells). #p<0.05 (AIP1 vs nifedipine, n=4 cells).
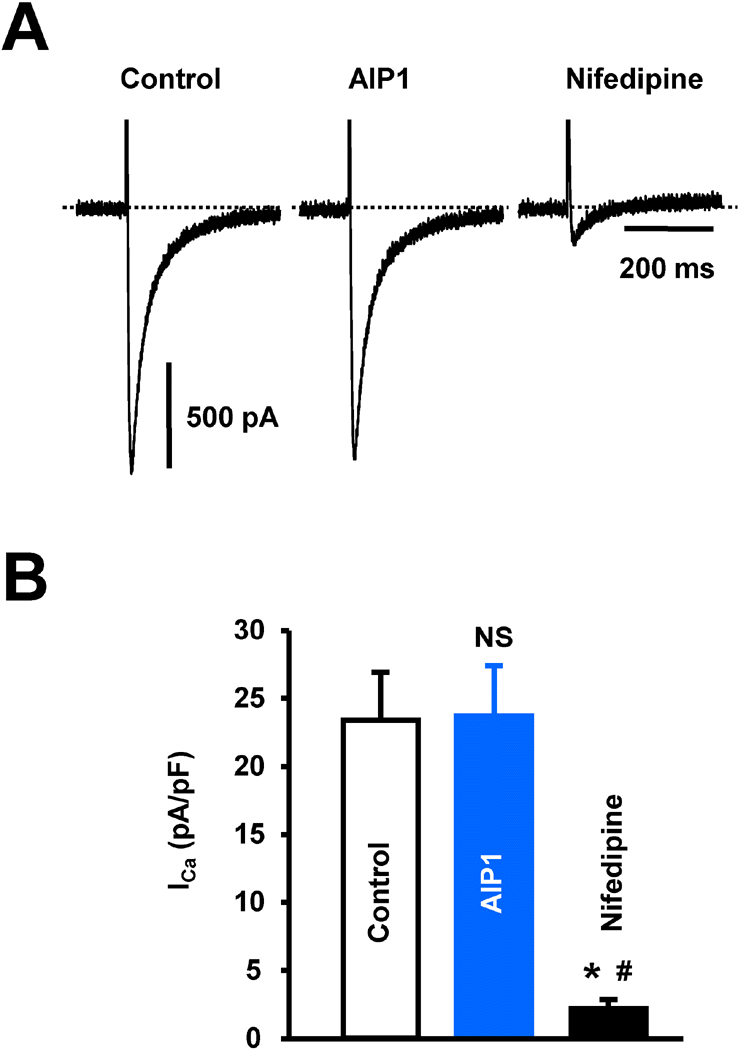
Fig. 4.
Effects of AIP1 on IKr and IKs. (A) Representative traces of action potentials obtained under control conditions, in the presence of 10 μM E-4031, and with additional application of 50 μg/ml AIP1. (B) Summary of APD50 as shown in (A). ∗p<0.05 (Control vs E-4031 + AIP1, n=6 cells). NS, not significant (E-4031 vs E-4031 + AIP1, n=6 cells). (C) The traces of action potentials obtained under control conditions, in the presence of 20 μM chromanol 293B, and with additional application of 50 μg/ml AIP1. (D) Summary of APD50 as shown in (C). ∗p<0.05 (Control vs chromanol 293B+AIP1, n=4 cells). #p<0.05 (chromanol 293B vs chromanol 293B + AIP1, n=4 cells).
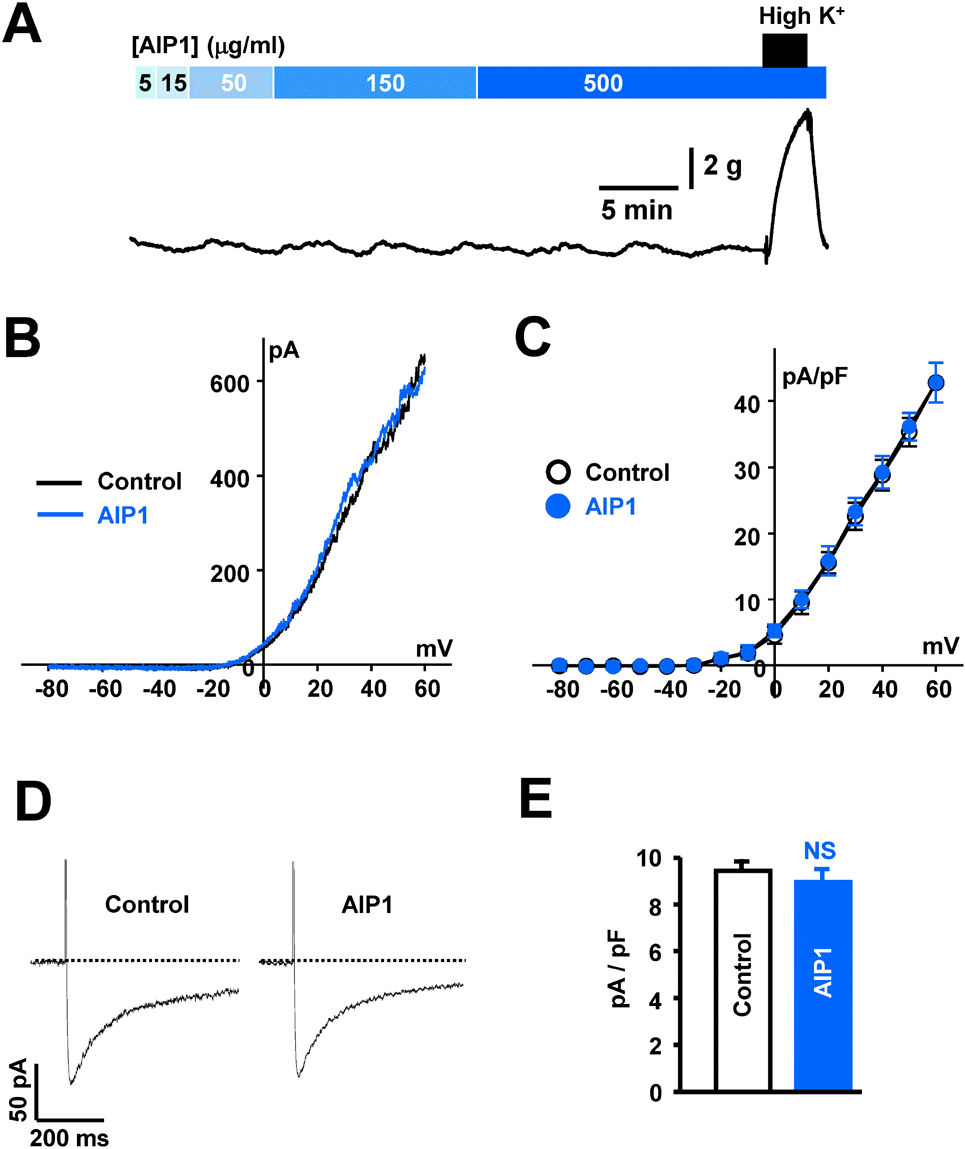
Fig. 5.
AIP1 did not affect coronary vascular tone or ion channels. (A) Representative traces showing the effect of various concentrations of AIP1 on vascular tone in coronary arteries. At the end of each experiment, 60 mM high K+ solution was applied to test the effectiveness of arteries (n=4 tissues). (B) Voltage-dependent K+ currents recorded using the ramp-pulse protocol (from –80 to + 60 mV for 280 ms) under control conditions (black line, n=5 cells) and in the presence of 50 μg/ml AIP1 (blue line, n=5 cells). (C) I-V relationship of voltage-dependent K+ currents obtained by step voltages (from –80 and +60 mV in steps of 10 mV) under control conditions (❍, n=5 cells) and in the presence of 50 μg/ml AIP1 (, n=5 cells). (D) vascular Ca2+ currents obtained by one step voltage (– 60 mV to 0 mV) under control condition and in the presence of 50 μg/ml AIP1. (E) Statistical summary of ICa at 0 mV. NS, not significant (Control vs AIP1, n=3 cells).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download