Abstract
Although anti-atherogenic effects of cilostazol have been suggested, its effects on the expression of SR in macrophages are unclear. This study investigated the role of cilostazol on CD36 expression of murine macrophages enhanced by HNE, a byproduct of lipid peroxidation. The stimulation of macrophages with HNE led to an increased expression of CD36, which was significantly attenuated by NAC, an antioxidant. Moreover, the increased production of ROS by HNE was completely abolished by NADPH oxidase inhibitors, DPI and apocynin, as well as by the 5-LO inhibitor, MK886, but not by inhibitors for other oxidases. This suggested that NADPH-oxidase and 5-LO were major sources of ROS induced by HNE. In addition, HNE-enhanced expression of CD36 was reduced by these inhibitors, which indicated a role for NADPH oxidase and 5-LO on CD36 expression. In our present study, cilostazol was a significant inhibitor of ROS production, as well as CD36 expression induced by HNE. An increase in NADPH oxidase activity by HNE was significantly attenuated by cilostazol, however cilostazol had no effect on HNE-enhanced 5-LO activity. Together, these results suggest that cilostazol attenuates HNE-enhanced CD36 expression on murine macrophages thorough inhibition of NADPH oxidase-derived ROS generation.
REFERENCES
Amer J., Goldfarb A., Fibach E. Flowcytometric measurement of reactive oxygen species production by normal and thalassaemic red blood cells. Eur J Haematol. 70:84–90. 2003.
Berliner JA., Navab M., Fogelman AM., Frank JS., Demer LL., Edwards PA., Watson AD., Lusis AJ. Atherosclerosis: basic mechanisms. Oxidation, inflammation, and genetics. Circulation. 91:2488–2496. 1995.
Cevik MO., Katsuyama M., Kanda S., Kaneko T., Iwata K., Ibi M., Matsuno K., Kakehi T., Cui W., Sasaki M., Yabe-Nishimura C. The AP-1 site is essential for the promoter activity of NOX1/NADPH oxidase, a vascular superoxide-producing enzyme: Possible involvement of the ERK1/2-JunB pathway. Biochem Biophys Res Commun. 379:351–355. 2008.

Esterbauer H., Schaur RJ., Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 11:81–128. 1991.

Forman HJ., Fukuto JM., Miller T., Zhang H., Rinna A., Levy S. The chemistry of cell signaling by reactive oxygen and nitrogen species and 4-hydroxynonenal. Arch Biochem Biophys. 477:183–195. 2008.

Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 294:1871–1875. 2001.

Greaves DR., Gordon S. Thematic review series: the immune system and atherogenesis. Recent insights into the biology of macro-phage scavenger receptors. J Lipid Res. 46:11–20. 2005.

Han J., Hajjar DP., Febbraio M., Nicholson AC. Native and modified low density lipoproteins increase the functional expression of the macrophage class B scavenger receptor. J Biol Chem. 272:21645–21659. 1997.
Ishii T., Itoh K., Ruiz E., Leake DS., Unoki H., Yamamoto M., Mann GE. Role of Nrf2 in the regulation of CD36 and stress protein expression in murine macrophages: activation by oxidatively modified LDL and 4-hydroxynonenal. Circ Res. 94:609–616. 2004.
Katsuyama M., Ozgur CM., Arakawa N., Kakehi T., Nishinaka T., Iwata K., Ibi M., Matsuno K., Yabe-Nishimura C. Myocyte enhancer factor 2B is involved in the inducible expression of NOX1/NADPH oxidase, a vascular superoxide-producing enzyme. FEBS J. 274:5128–5136. 2007.

Kimura Y., Tani T., Kanbe T., Watanabe K. Effect of cilostazol on platelet aggregation and experimental thrombosis. Arzneimittelforschung. 35:1144–1149. 1985.
Knöbel Y., Glei M., Osswald K., Pool-Zobel BL. Ferric iron increases ROS formation, modulates cell growth and enhances genotoxic damage by 4-hydroxynonenal in human colon tumor cells. Toxicol In Vitro. 20:793–800. 2006.

Kumagai T., Matsukawa N., Kaneko Y., Kusumi Y., Mitsumata M., Uchida K. A lipid peroxidation-derived inflammatory mediator: identification of 4-hydroxy-2-nonenal as a potential inducer of cyclooxygenase-2 in macrophages. J Biol Chem. 279:48389–48396. 2004.
Lee JH., Oh GT., Park SY., Choi JH., Park JG., Kim CD., Lee WS., Rhim BY., Shin YW., Hong KW. Cilostazol reduces atherosclerosis by inhibition of superoxide and tumor necrosis factor-formation in low-density lipoprotein receptor-null mice fed high cholesterol. J Pharmacol Exp Ther. 313:502–509. 2005.
Lee JY., Jung GY., Heo HJ., Yun MR., Park JY., Bae SS., Hong KW., Lee WS., Kim CD. 4-Hydroxynonenal induces vascular smooth muscle cell apoptosis through mitochondrial generation of reactive oxygen species. Toxicol Lett. 166:212–221. 2006.

Leonarduzzi G., Chiarpotto E., Biasi F., Poli G. 4-Hydroxynonenal and cholesterol oxidation products in atherosclerosis. Mol Nutr Food Res. 49:1044–1049. 2005.

Manea A., Manea SA., Gafencu AV., Raicu M. Regulation of NADPH oxidase subunit p22 (phox) by NF-kB in human aortic smooth muscle cells. Arch Physiol Biochem. 113:163–172. 2007.
Moore KJ., Freeman MW. Scavenger receptors in atherosclerosis: beyond lipid uptake. Arterioscler Thromb Vasc Biol. 26:1702–1711. 2006.
Nakata A., Nakagawa Y., Nishida M., Nozaki S., Miyagawa J., Nakagawa T., Tamura R., Matsumoto K., Kameda-Takemura K., Yamashita S., Matsuzawa Y. CD36, a novel receptor for oxidized low-density lipoproteins, is highly expressed on lipid-laden macrophages in human atherosclerotic aorta. Arterioscler Thromb Vasc Biol. 19:1333–1339. 1999.

Okutsu R., Yoshikawa T., Nagasawa M., Hirose Y., Takase H., Mitani K., Okada K., Miyakoda G., Yabuuchi Y. Cilostazol inhibits modified low-density lipoprotein uptake and foam cell formation in mouse peritoneal macrophages. Atherosclerosis. 2008. Nov 17. [Epub ahead of print].

Peters-Golden M., Brock TG. 5-lipoxygenase and FLAP. Prostaglandins Leukot Essent Fatty Acids. 69:99–109. 2003.

Raza H., John A. 4-hydroxynonenal induces mitochondrial oxidative stress, apoptosis and expression of glutathione S-transferase A4-4 and cytochrome P450 2E1 in PC12 cells. Toxicol Appl Pharmacol. 216:309–318. 2006.

Samuelsson B., Dahlen SE., Lindgren JA., Rouzer CA., Serhan CN. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 237:1171–1176. 1987.

Sampey BP., Carbone DL., Doorn JA., Petersen DR. 4-Hydroxy-2-nonenal adduction of extracellular signal-regulated kinase (Erk) and the inhibition of hepatocyte Erk-Est-like protein-1-activating protein-1 signal transduction. Mol Pharmacol. 71:871–883. 2007.

Serezani CH., Aronoff DM., Jancar S., Peters-Golden M. Leukotriene B4 mediates p47phox phosphorylation and membrane translocation in polyunsaturated fatty acid-stimulated neutrophils. J Leukoc Biol. 78:976–984. 2005.
Serezani CH., Aronoff DM., Jancar S., Mancuso P., Peters-Golden M. Leukotrienes enhance the bactericidal activity of alveolar macrophages against Klebsiella pneumoniae through the activation of NADPH oxidase. Blood. 106:1067–1075. 2005.

Shimizu T., Radmark O., Samuelsson B. Enzyme with dual lipoxygenase activities catalyzes leukotriene A4 synthesis from arachidonic acid. Proc Natl Acad Sci USA. 81:689–693. 1984.

Shin HK., Kim YK., Kim KY., Lee JH., Hong KW. Remnant lipoprotein particles induce apoptosis in endothelial cells by NAD(P)H oxidase-mediated production of superoxide and cytokines via lectin-like oxidized low-density lipoprotein receptor-1 activation: Prevention by cilostazol. Circulation. 109:1022–1028. 2004.
Stocker R., Keaney JF Jr. Role of oxidative modifications in atherosclerosis. Physiol Rev. 84:1381–1478. 2004.

Thannickal VJ., Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 279:L1005–1028. 2000.

Uchida K. 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog Lipid Res. 42:318–343. 2003.

Woo CH., You HJ., Cho SH. Leukotriene B(4) stimulates Rac-ERK cascade to generate reactive oxygen species that mediates chemotaxis. J Biol Chem. 277:8572–8578. 2002.

Yesner LM., Huh HY., Pearce SF., Siverstein RL. Regulation of monocyte CD36 and thrombospondin-1 expression by soluble mediators. Arterioscler Thromb Vasc Biol. 16:1019–1025. 1996.

Yoshida H., Quehenberger O., Kondratenko N., Green S., Steinberg D. Minimally oxidized low-density lipoproteinincreases expression of scavenger receptor A, CD36, and macrosialin in resident mouse peritoneal macrophages. Arterioscler Thromb Vasc Biol. 18:794–802. 1998.
Fig. 1.
Effect of HNE on the expression of CD36 in J774A.1 macrophages. (A) Total RNA was extracted from macrophages treated with 10 μM HNE for the indicated time (0 ~ 18 hrs). 2 μg RNA was subjected to RT-PCR. Band intensity for CD36 mRNA was quantified by densitometric scanning and normalized to that of GAPDH. (B) Macrophages were incubated with 10 μM HNE, and cellular lysates were analyzed by Western blotting using anti-CD36 antibody. β-actin as used to normalize density. Each photograph is the representative of 5 independent experiments. Data is presented as mean±SE from 5 independent experiments. ∗∗p<0.01 vs. value at time 0.
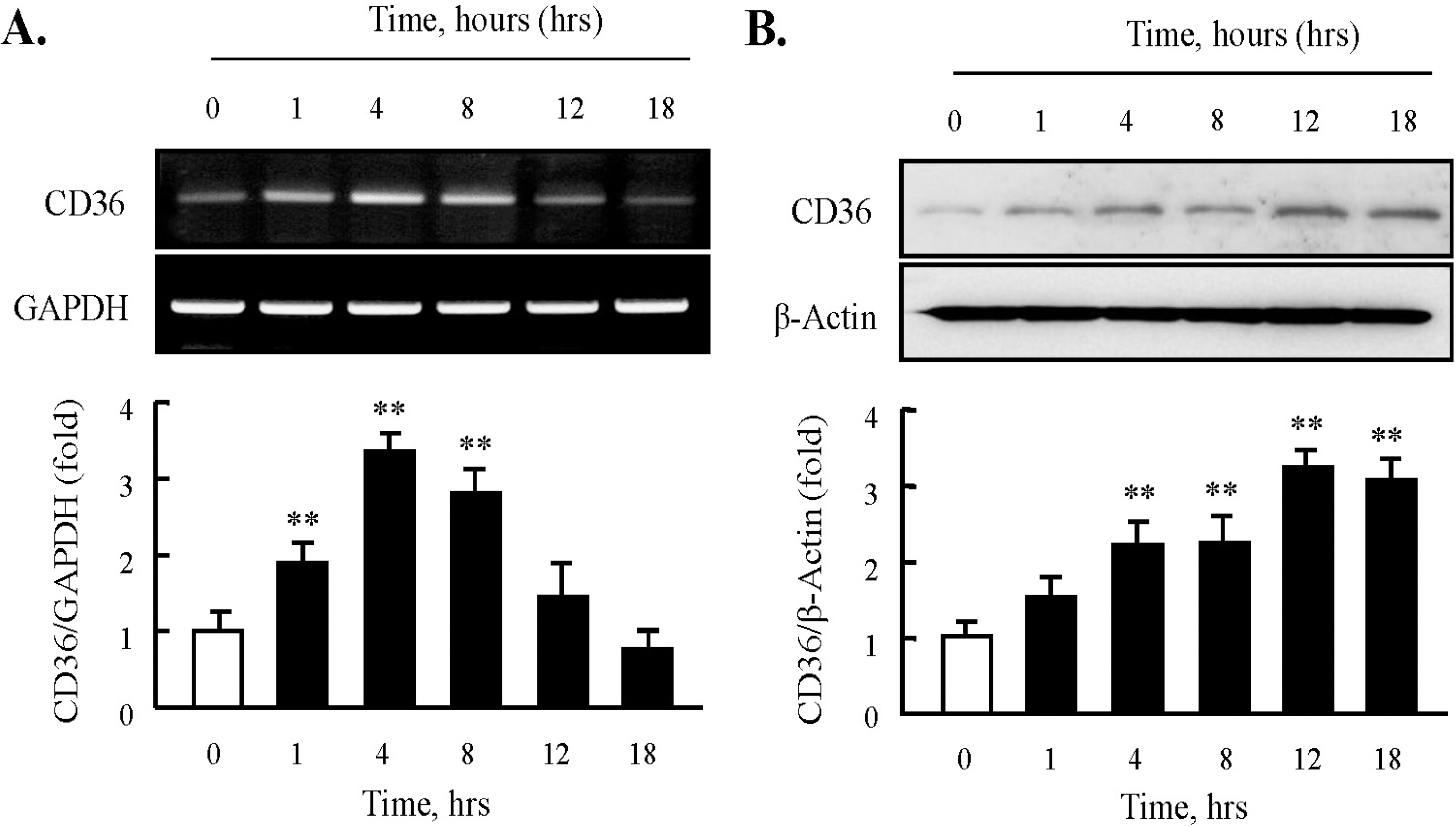
Fig. 2.
Role of ROS on HNE-induced CD36 expression in J774A.1 macrophages. Macrophages were treated with 10 μM HNE for the indicated time in the absence or presence of N-acetylcysteine (Nac, 5 mM). The levels of CD36 mRNA and protein were determined by RT-PCR (A) and Western blot analysis (B), respectively. Each fig is the representative of 4 independent experiments. Data was presented as mean±SE from 5 independent experiments. ∗∗p<0.01 vs. control; ##p<0.01 vs. veh (vehicle).
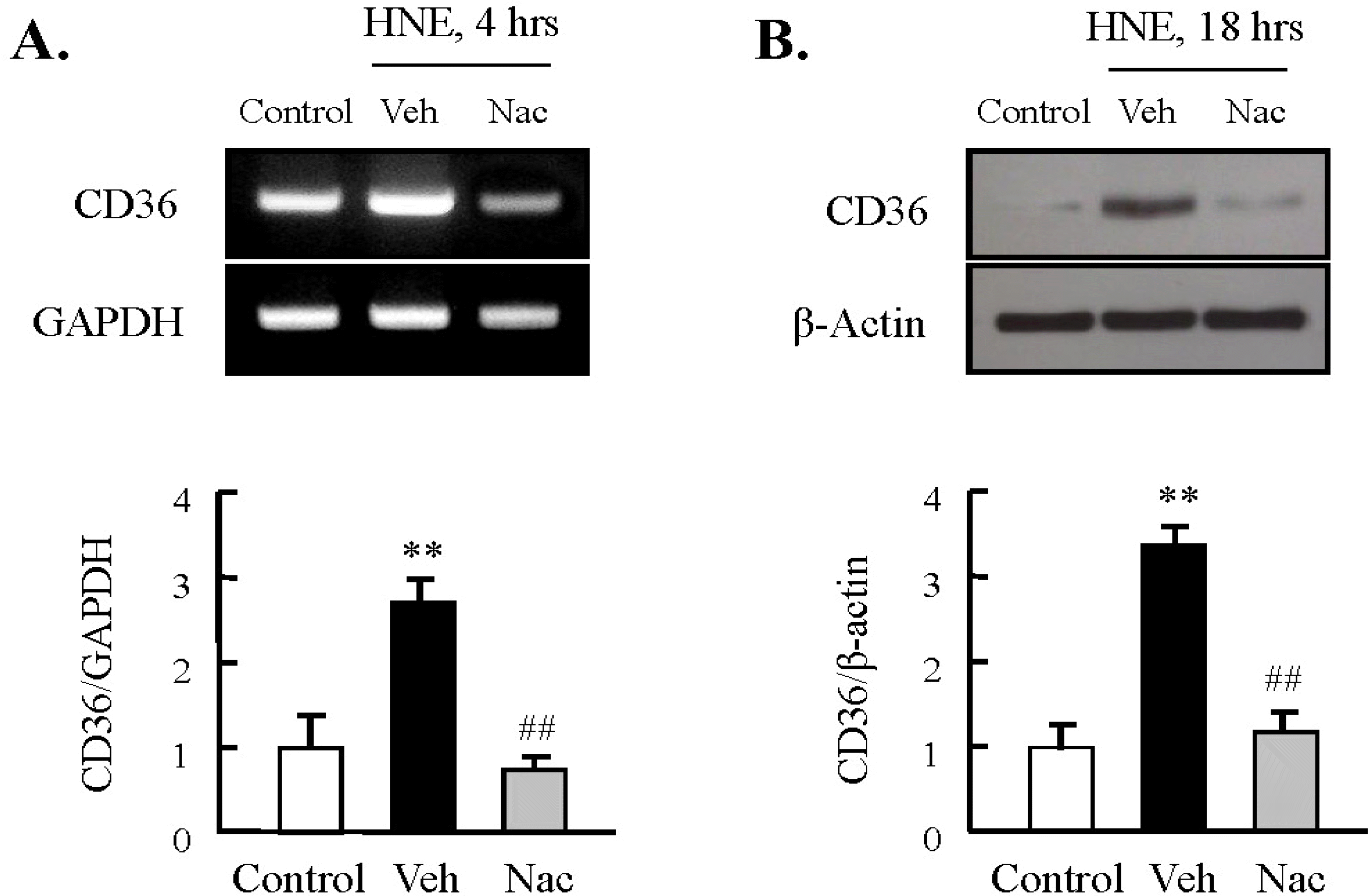
Fig. 3.
Effects of inhibitors of NADPH oxidase or lipoxygenase on HNE-induced ROS production in J774A.1. macrophages. (A) Cells were treated with different concentrations of HNE for 45 min, and then ROS generation was measured by flow cytometry (FACS) using DCF fluorescence. Fluorescence intensity was analyzed by CellQuest Software. Data was presented as mean±SE from 5 independent experiments. ∗∗p<0.01 vs. value at concentration 0. (B) Cells were incubated with 10 μM HNE for 45 min after pre-treatment with various inhibitors for ROS including N-acetylcysteine (Nac, 5 mM), diphenylamine iodonium (DPI, 10 μM), apocynine (Apo, 300 μM), nordihydroguaiaretic acid (NDGA, 10 μM), allopurinol (Allo, 100 μM), or rotenone (Ro, 1 μM) plus stigmatellin (Stig, 1 μM) for 30 min. (C) Cells were pre-treated with eicosanoid inhibitors including MK886 (10 μM), baicalein (10 μM), or indomethacin (Indo, 100 μM) for 30 min, and then stimulated with 10 μM HNE for 45 min. ROS generation was analyzed by FACS. Data was presented as mean±SE from 5 independent experiments. ∗∗p<0.01 vs. control; ##p<0.01 vs. veh (vehicle).
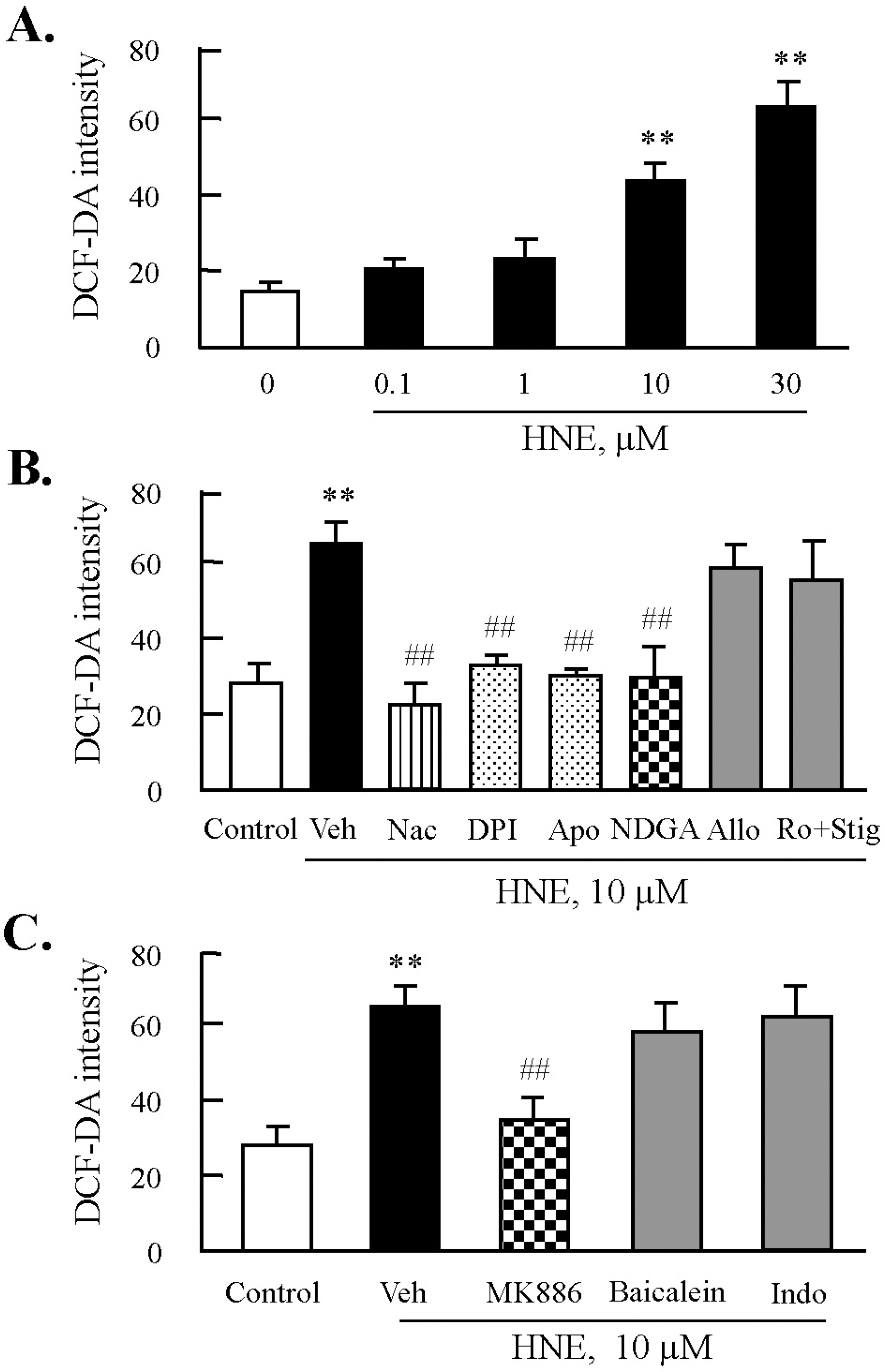
Fig. 4.
Effect of inhibitors for NADPH oxidase or 5-lipoxygenase on HNE-induced CD36 expression in J774A.1. macrophages. Macrophages were treated with diphenylamine iodonium (DPI, 10 μM), apocynin (Apo, 300 μM), or MK886 (10 μM) for 30 min prior to HNE application. Cells were stimulated with 10 μM HNE for the indicated time. The levels of CD36 mRNA and protein were determined by RT-PCR (A) and Western blot analysis (B), respectively. Each fig is the representative of 5 independent experiments. Data was presented as mean±SE from 5 independent experiments. ∗∗p<0.01 vs. control; #p<0.05; ##p<0.01 vs. veh (vehicle).
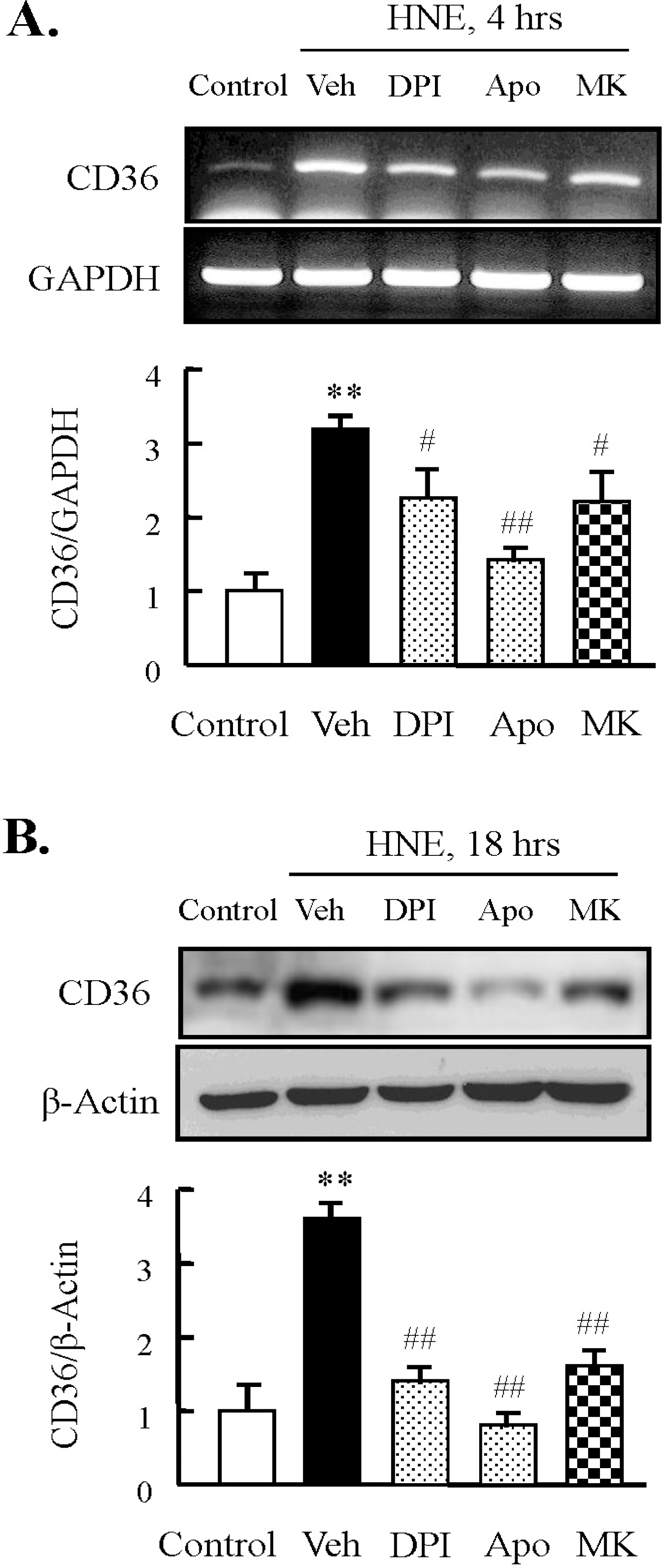
Fig. 5.
Effects of cilostazol on the expression of CD36 in J774A.1. macrophages. Effects of cilostazol on the expression of CD36 mRNA (A) and protein (B) were analyzed using RT-PCR and Western blot, respectively. Each fig is the representative of 4 independent experiments. Data was presented as mean±SE from 4 independent experiments. ∗∗p<0.01 vs. control; ##p<0.01 vs. HNE alone.
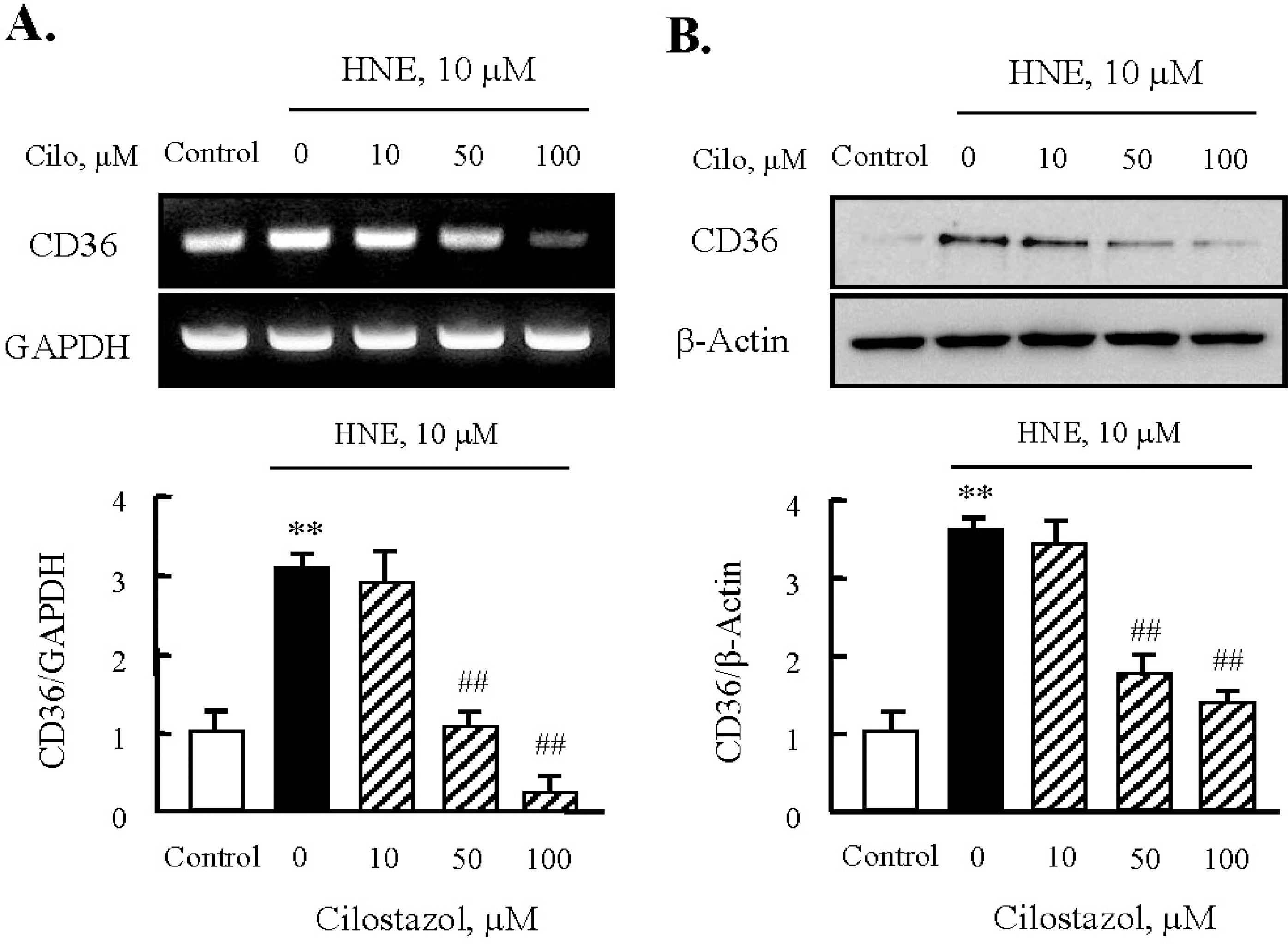
Fig. 6.
Effects of cilostazol on the generation of ROS in J774A.1. macrophages. After pre-treatment of various concentrations of cilostazol, macrophages were stimulated with 10 μM HNE for 45 min. ROS generation was measured by flow cytometry (FACS) using DCF fluorescence. Data was presented as mean±SE from six independent experiments. ∗∗p<0.01 vs control; #p<0.05; ##p<0.01 vs. HNE alone.
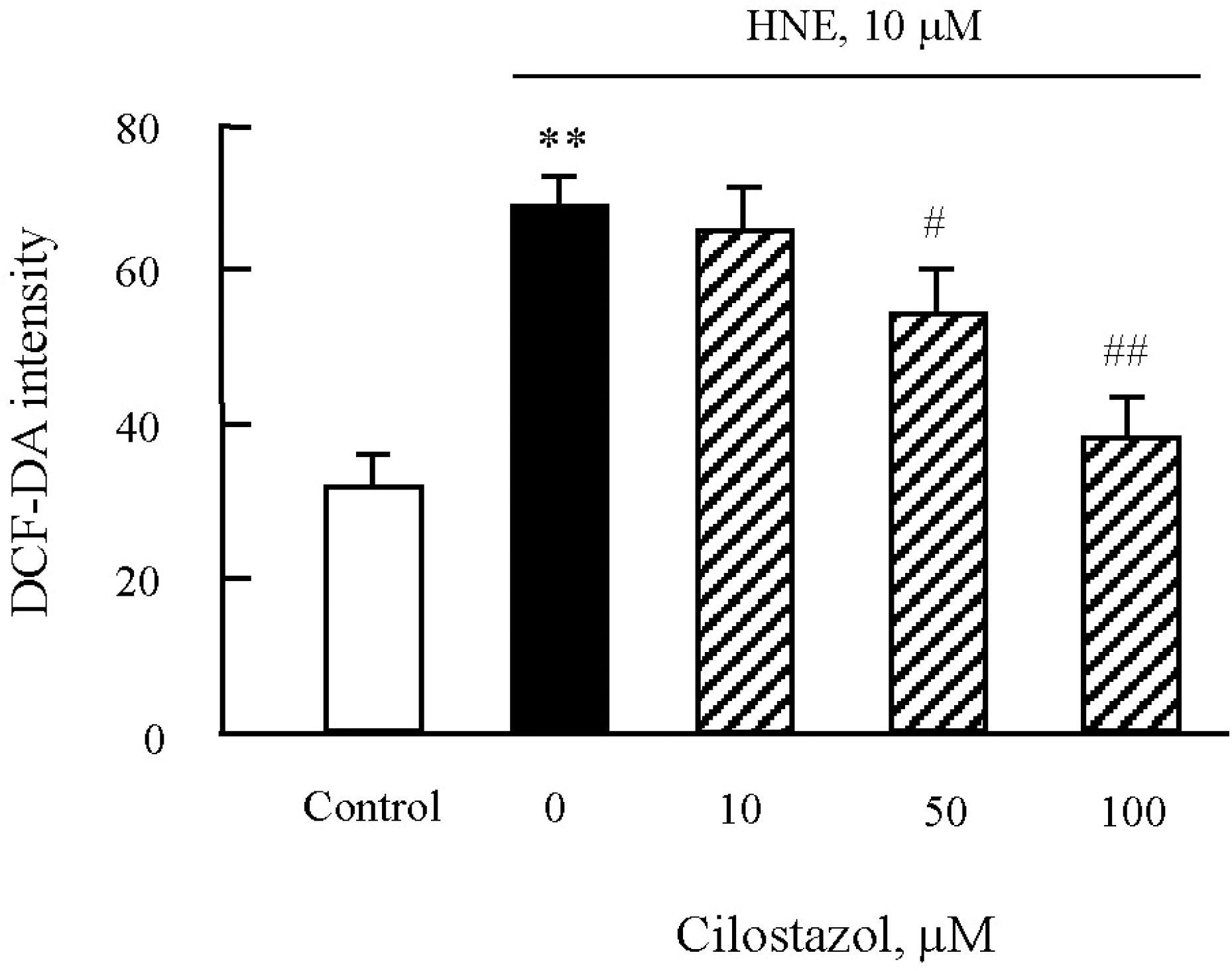
Fig. 7.
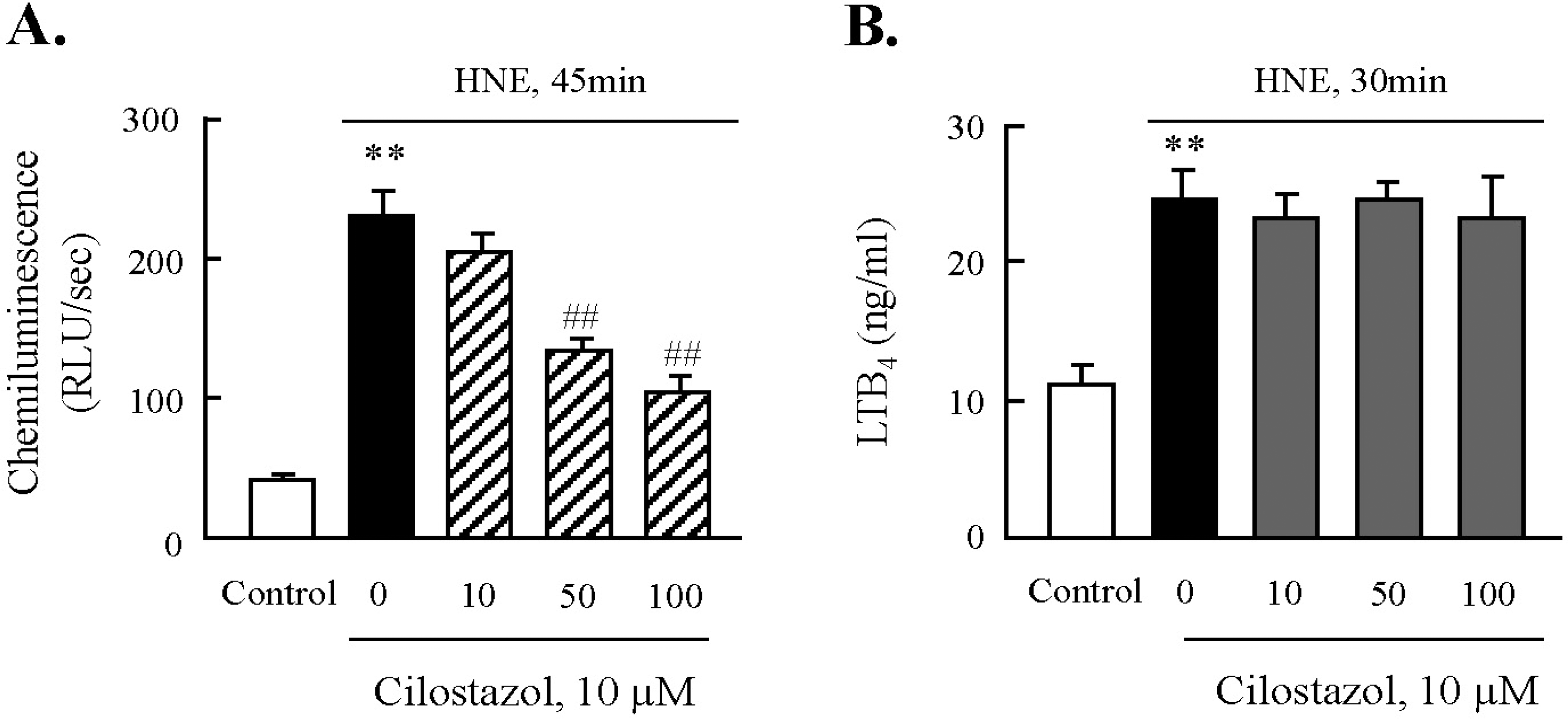
Effect of cilostazol on the activities of NADPH oxidase and 5-lipoxygenase. After pre-treatment with various concentrations of cilostazol, macrophages were stimulated with 10 μM HNE for the indicated time. The activities of NADPH oxidase (A) and 5-LO (B) were quantified by chemiluminescence assay and ELISA, respectively. Data was presented as mean±SE from 5 independent experiments. ∗∗p<0.01 vs. control; ##p<0.01 vs. HNE alone.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download