Abstract
Puerariae flos (PF) is a traditional oriental medicinal plant and has clinically been prescribed for a long time. The purpose of the present study was to examine the effect of PF on repeated stress-induced alterations of learning and memory on a Morris water maze (MWM) test in ovariectomized (OVX) female rats. The changes in the reactivity of the cholinergic system were assessed by measuring the immunoreactive neurons of choline acetyltransferase (ChAT) in the hippocampus after behavioral testing. The female rats were randomly divided into four groups: the nonoperated and nonstressed group (normal), the sham-operated and stressed group (control), the ovariectomized and stressed group (OS), and the ovariectomized, stressed and PF treated group (OSF). Rats were exposed to immobilization stress (IMO) for 14 d (2 h/d), and PF (400 mg/kg, p.o.) was administered 30 min before IMO stress. Results showed that treatments with PF caused significant reversals of the stress-induced deficits in learning and memory on a spatial memory task, and also increased the ChAT immunoreactivities. In conclusion, administration of PF improved spatial learning and memory in OVX rats, and PF may be useful for the treatment of postmenopausal-related dementia.
REFERENCES
Bammer G., Chesher GB. An analysis of some effects of ethanol on performance in a passive avoidance task. Psychopharmacology. 77:66–73. 1982.

Bremner JD., Narayan M., Anderson ER., Staib LH., Miller HL., Charney DS. Hippocampal volume reduction in major depression. Am J Psychiatry. 157:115–117. 2000.

Chen G., Chen KS., Knox J., Inglis J., Bernard A., Martin SJ., Justice A., McConlogue L., Games D., Freedman SB., Morris RG. A learning deficit related to age and β-amyloid plaques in a mouse model of Alzheimer's disease. Nature. 408:975–979. 2000.

Fibiger HC., Damsma G., Day JC. The Basal Forebrain. 1st ed.Plenum Press;New York: 1991.
Gur RC., Mozley PD., Resnick SM., Gottlieb GL., Kohn M., Zimmerman R., Herman G., Atlas S., Grossman R., Berretta D., Erwin R., Gur RE. Gender difference in age effect on brain atrophy measured by magnetic resonance imaging. Proc Natl Sci U S A. 88:2845–2849. 1991.
Hooge R., Dedeyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev. 36:60–90. 2001.
Janowsky DS., Overstreet DH. Psychopharmacology. The Fourth Generation of Progress. Raven Press, New York. 1995.
Kellendonk C., Gass P., Kretz O., Schutz G., Tronche F. Corticosteroid receptors in the brain: Gene targeting studies. Brain Res Bull. 57:73–83. 2002.

Luine VN., Jacome LF., Maclusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 144:2836–2844. 2003.

Matsunaga K., Mukasa M. The effect of alcohol on the human memory. Nihon Arukoru Yakubutsu Igakkai Zasshi. 21:64–73. 1986.
McEwen BS. From molecules to mind: stress, individual differences, and the social environment. Ann NY Acas Sci. 935:42–49. 2001a.
McEwen BS. Invited review: estrogen's effects on the brain: multiple sites and molecular mechanisms. J Appl Physiol. 91:2789–2801. 2001b.
Mcgonigal G., Thomas B., Mcquade C., Starr JM., Maclennam WJ., Whalley LJ. Epidemiology of Alzheimer's presentnile dementia in Scotland. Br Med J. 306:680–683. 1974-1988.
Nancy LD., William BL. Ovarian steroidal control of connectivity in the female hippocampus: an overview of recent experimental finding and speculations on its functional consequences. Hippocampus. 7:239–245. 1997.
Ohkura T., Isse K., Watabe H., Segawa Y., Mitsuya K., Enomoto H., Hayashi M., Yaoi Y. A clinical study on memory function in climacteric and periclimacteric women. Acta Obstetrics and Gynaecology. 46:271–276. 1994.
Paxinos G., Watson C., Pennisi M. Bregma, lamda and the interaural midpoint in stereotaxic surgery with rats of different sex. Strain and Weight. 13:139–143. 1985.
Rabbani O., Panickar KS., Rajakumar G., King MA., Bodor N., Meyer EM., Simpkins JW. 17β-Estradiol attenuates fimbrial lesion-induced decline of ChAT-immunoreactive neurons in the rat medial septum. Exp Neurol. 146:179–186. 1997.
Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension. 37:1199–1208. 2001.

Sapolsky RM. The possibility of neurotoxicity in the hippocampus in major depression: a primer on neuron death. Biol Psychiatry. 48:755–765. 2000.

Sato T., Teramoto T., Tanaka K., Ohnishi Y., Irifune M., Nishikawa T. Effects of ovariectomizy and calcium deficiency on learning and memory of eight-arm radial maze in middle-aged female rats. Behav Brain Res. 142:207–216. 2003.
Saunders- Pullman R., Gordon-Elliott J., Parides M., Fahn S., Saunders HR., Bressman S. The effect of estrogen replacement on early Parkinson's disease. Neurology. 52:1417–1421. 1999.

Sheline YI. 3D MRI studies of neuroanatomic changes in unipolar major depression: the role of stress and medical comorbidity. Biol Psychiatry. 48:791–800. 2000.

Shors TJ., Leuner B. Estrogen-mediated effects in depression and memory formation in females. J Affective Disorders. 74:85–96. 2003.
Wolly CS. Effects of estrogen in the CNS. Curr Opin Neurobiol. 9:755–765. 2000.
Wu X., Glinn MA., Ostrowski NL., Su Y., Ni B., Cole HW., Bryant HU., Paul SM. Raloxifene and estradiol benzonate both fully restore hippocampal choline acetyltransferase activity in ovariectomized rats. Brain Res. 847:98–104. 1999.
Xu J., Zhu J., Shi C., Guo K., Yew DT. Effects of genistein on hippocampal neurodegeneration of ovariectomized rats. J Mol Neurosci. 31:101–112. 2007.

Yamazaki T., Hosono T., Matsushita Y., Kawashima K., Someya M., Nakahima Y., Narui K., Hibi Y., Ishizaki M., Kinjo J., Nohara T. Pharmacological studies on Puerariae flos IV: effects of pueraria thomsonii dried flower extracts on blood ethanol and acetaldehyde levels in humans. Int J Clin Pharmacol Res. 22:23–28. 2002.
Fig. 1.
Changes of the latency time during 6 d of the acquisition test in the Morris water maze test. Repeated measures of ANOVA of swimming time among the groups, followed by LSD test. Each value represents mean±S.E.M.
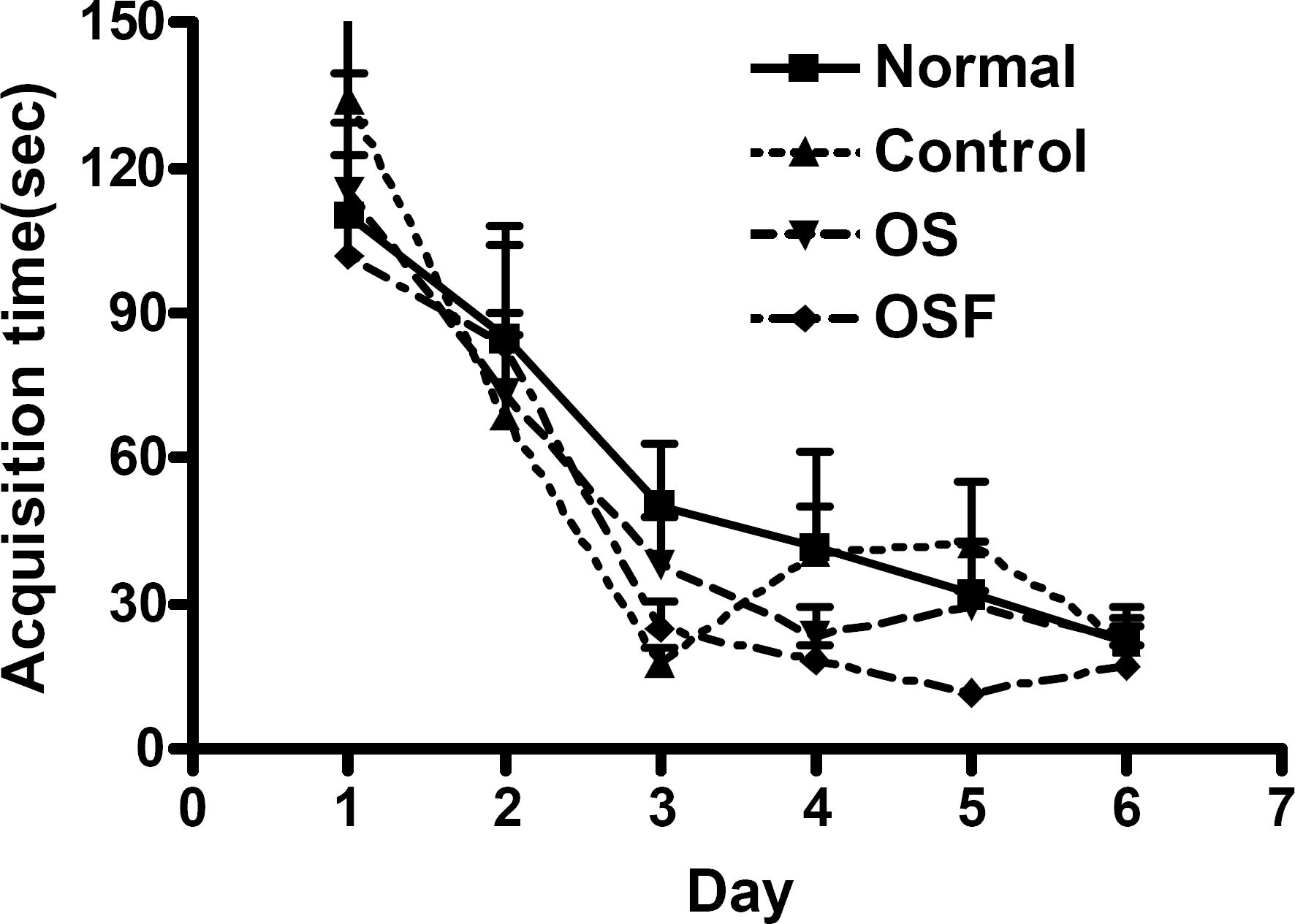
Fig. 2.
The latency time on the 7th day of the retention test in the Morris water maze test. The results of retention test were analyzed by performing separate measures of one-way ANOVA of swimming time among the groups, followed by LSD test. Each value represents mean±S.E.M. #p<0.05 and ##p<0.01 compared to OS.
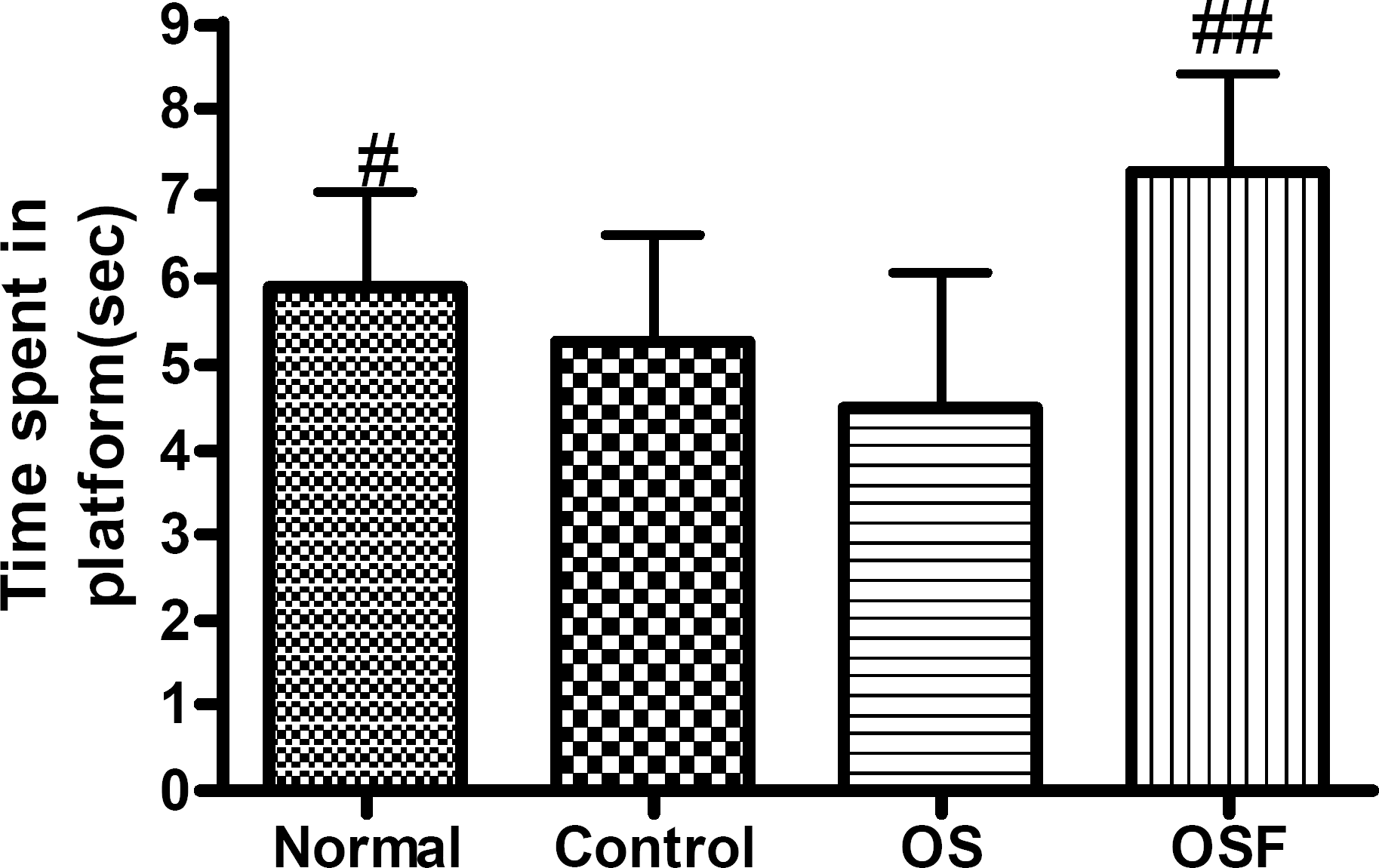
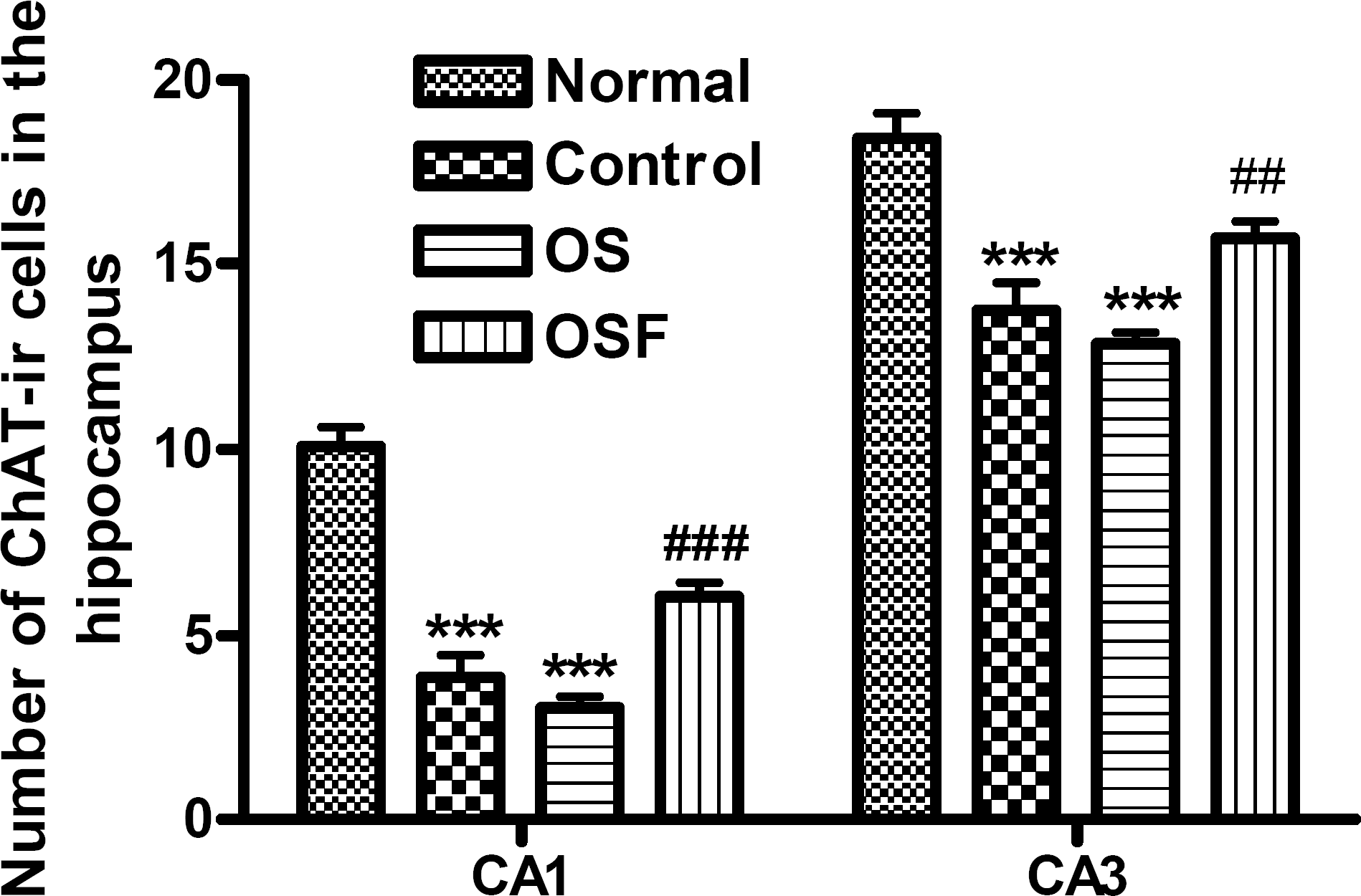
Fig. 3.
The number of choline acetyltransferase (ChAT) immunostained nuclei in the different hippocampal areas of the experimental groups after 7 d of the water maze test. The results of ChAT-reactivity were analyzed by performing separate one-way ANOVA of neurons among the groups, followed by LSD test. Each value represents mean±S.E.M. ∗∗∗p<0.01 compared to normal and ##p<0.01, ###p<0.001 compared to OS.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download