Abstract
A series of substituted 2-arylnaphthyridin-4-one analogues, which were previously synthesized in our laboratory, were evaluated for their in vitro cytotoxic activity against human lung cancer A549 and human renal cancer Caki-2 cells using MTT assay. Some compounds (11, 12, and 13) showed stronger cytotoxicity than colchicine against both tumor cell lines, and compound 13 exhibited the most potent activity with IC50 values of 2.3 and 13.4 μM, respectively. Three-dimensional quantitative structure activity relationship (3D-QSAR) studies of comparative molecular field analysis (CoMFA) and comparative molecular similarity indices analysis (CoMSIA) were performed. Predictive 3D-QSAR models were obtained with q2 values of 0.869 and 0.872 and r2ncv values of 0.983 and 0.993 for CoMFA and CoMSIA, respectively. These results demonstrate that CoMFA and CoMSIA models could be reliably used in the design of novel cytotoxic agents.
References
Chen K., Kuo SC., Hsieh MC., Mauger A., Lin CM., Hamel E., Lee KH. Antitumor agents. 174. 2′,3′,4′,5,6,7-Substituted 2-phenyl-1,8-naphthyridin-4-ones: their synthesis, cytotoxicity, and inhibition of tubulin polymerization. J Med Chem. 40:2266–2275. 1997.

Chung ML. Synthesis and Anti-Cancer Activity of 2-Phenyl-1,8-naphthyridine-4-one Derivatives. Chung Ang Graduate School Master thesis. 2004.
Hamel E., Lin CM., Plowman J., Wang HK., Lee KH., Paull KD. Antitumor 2,3-Dihydro-2-(aryl)-4(1H)-quinazolinone Derivatives. Interactions with Tubulin. Biochem Pharmacol. 51:53–59. 1996.
Hastie SB. Interaction of colchicine with tubulin. Pharmacol Ther. 51:377–401. 1991.
Hour MJ., Huang LJ., Kuo SC., Xia Y., Bastow K., Nakanishi Y., Hamel E., Lee KH. 6-Alkylamino- and 2,3-dihydro-3′-methoxy-2-phenyl-4-quinazolinones and related compounds: their synthesis, cytotoxicity, and inhibition of tubulin polymerization. J Med Chem. 43:4479–4487. 2000.

Jackson PL., Scott KR., Southerland WM., Fang YY. Enaminones 8: CoMFA and CoMSIA studies on some anticonvulsant enaminones. Bioor Med Chem. 17:133–140. 2009.

Kuo SC., Lee HZ., Juang JP., Lin YT., Wu TS., Chang JJ., Lednicer D., Paull KD., Lin CM., Hamel E., Lee KH. Synthesis and cytotoxicity of 1,6,7,8-substituted 2-(4'-substituted-phenyl)-4-quinolones and related compounds: Identification as antimitotic agents interacting with tubulin. J Med Chem. 36:1146–1156. 1993.
Lai YY., Huang LJ., Lee KH., Xiao Z., Bastow KF., Yamori T., Kuo SC. Synthesis and biological relationships of 3′,6-substituted 2-phenyl-4-quinolone-3-carboxylic acid derivatives as antimitotic agents. Bioorg Med Chem. 13:265–275. 2005.

Li L., Wang HK., Kuo SC., Wu TS., Lednicer D., Lin CM., Hamel E., Lee KH. Antitumor agents. 150. 2′,3′,4′,5′,5,6,7-Substituted 2-phenyl-4-quinolones and related compounds: Their synthesis, cytotoxicity, and inhibition of tubulin polymerization. J Med Chem. 37:1126–1135. 1994.

Manthey JA., Guthrie N. Antiproliferative Activities of Citrus Flavonoids against Six Human Cancer Cell Lines. J Agric Food Chem. 50:5837–5843. 2002.

Manthey JA., Guthrie N., Grohmann K. Biological properties of citrus flavonoids pertaining to cancer and inflammation. Curr Med Chem. 8:135–153. 2001.

Nakamura S., Kozuka M., Bastow KF., Tokuda H., Nishino H., Suzuki M., Tatsuzaki J., Morris Natschke SL., Kuo SC., Lee KH. Cancer preventive agents, part 2: synthesis and evaluation of 2-phenyl-4-quinolone and 9-oxo-9,10-dihydroacridine derivatives as novel antitumor promoters. Bioorg Med Chem. 13:4396–4401. 2005.

Park JG., Kramer BS., Steinberg SM., Carmichael J., Collins JM., Minna JD., Gazdar AF. Chemosensitivity testing of human colorectal carcinoma cell lines using a tetrazolium-based colorimetric assay. Cancer research. 47:5875–5879. 1987.
Rieger JM., Brown ML., Sullivan GW., Linden J., Macdonald TL. Design, Synthesis, and Evaluation of Novel A2A Adenosine Receptor Agonists. J Med Chem. 44:531–539. 2001.

Rowinsky EK., Donehower RC. The clinical pharmacology and use of antimicrotubule agents in cancer chemotherapeutics. Pharmacol Ther. 52:35–84. 1992.

Shi Q., Chen K., Li L. Antitumor Agents. 154. Cytotoxic and antimitotic flavonols from Polanisia dodecandra. J Nat Prod. 58:475–482. 1995.

SYBYL Molecular Modeling Software. Tripos Inc., St. Louis, USA. 2009.
Verwij J., Clavel M., Chevalier B. Paclitaxel (Taxol) and docetaxel (Taxotere): Not simply two of a kind. Ann Oncol. 5:495–505. 1994.
Xia Y., Yang ZY., Hour MJ., Kuo SC., Xia P., Bastow KF., Nakanishi Y., Nampoothiri P., Hackl T., Hamel E., Lee K H. Antitumor agents. Part 204: Synthesis and biological evaluation of substituted 2-arylquinazolinones. Bioorg Med Chem Lett. 11:1193–1196. 2001.
Fig. 1.
Scatter plot of actual activities versus predicted activities against A549 (A) and Caki-2 (B) tumor cell lines (: training set molecules, ▴: test set molecules).
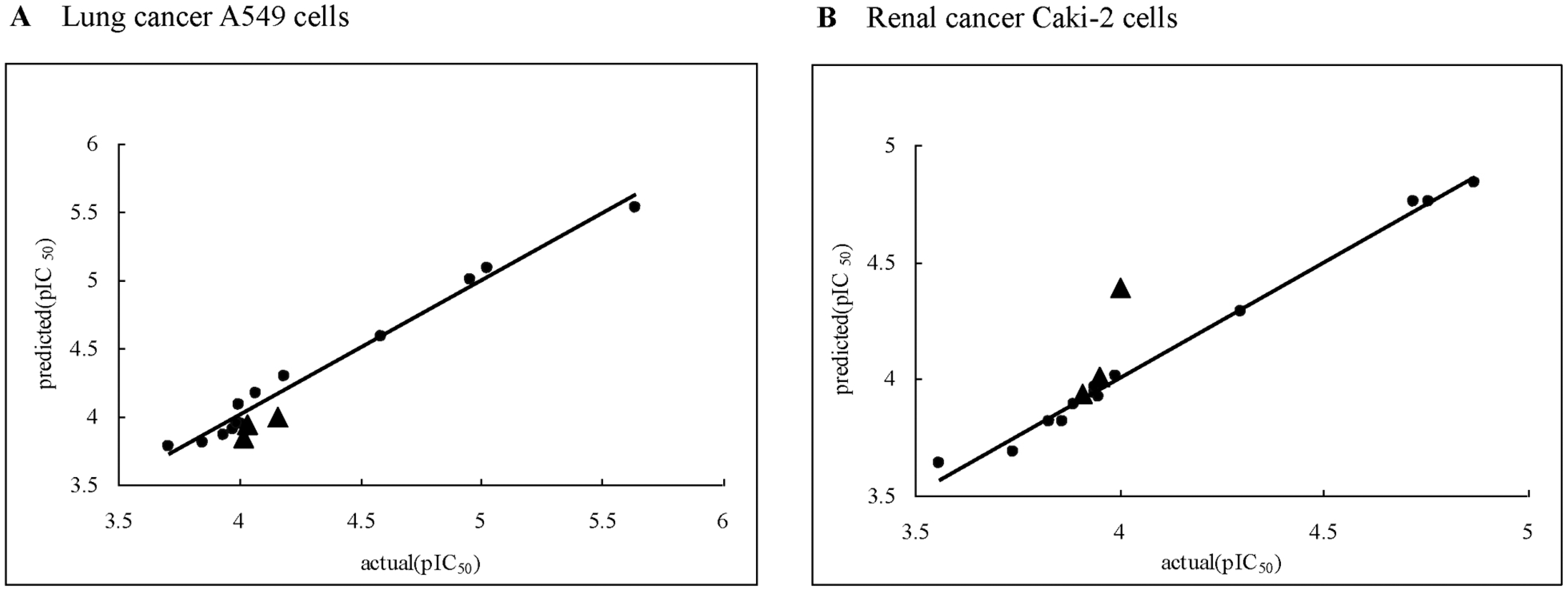
Fig. 2.
Contour maps of steric and electrostatic fields in lung cancer A549 (A) and renal cancer Caki-2 (B) cells. Compound 13 is shown inside the field. Color-coding is as follows: Green indicates regions where bulky groups increase activity, whereas yellow indicates regions where bulky groups decrease activity. Blue indicates that positive charge favors high activity, whereas red indicates that negative charge favors high activity.
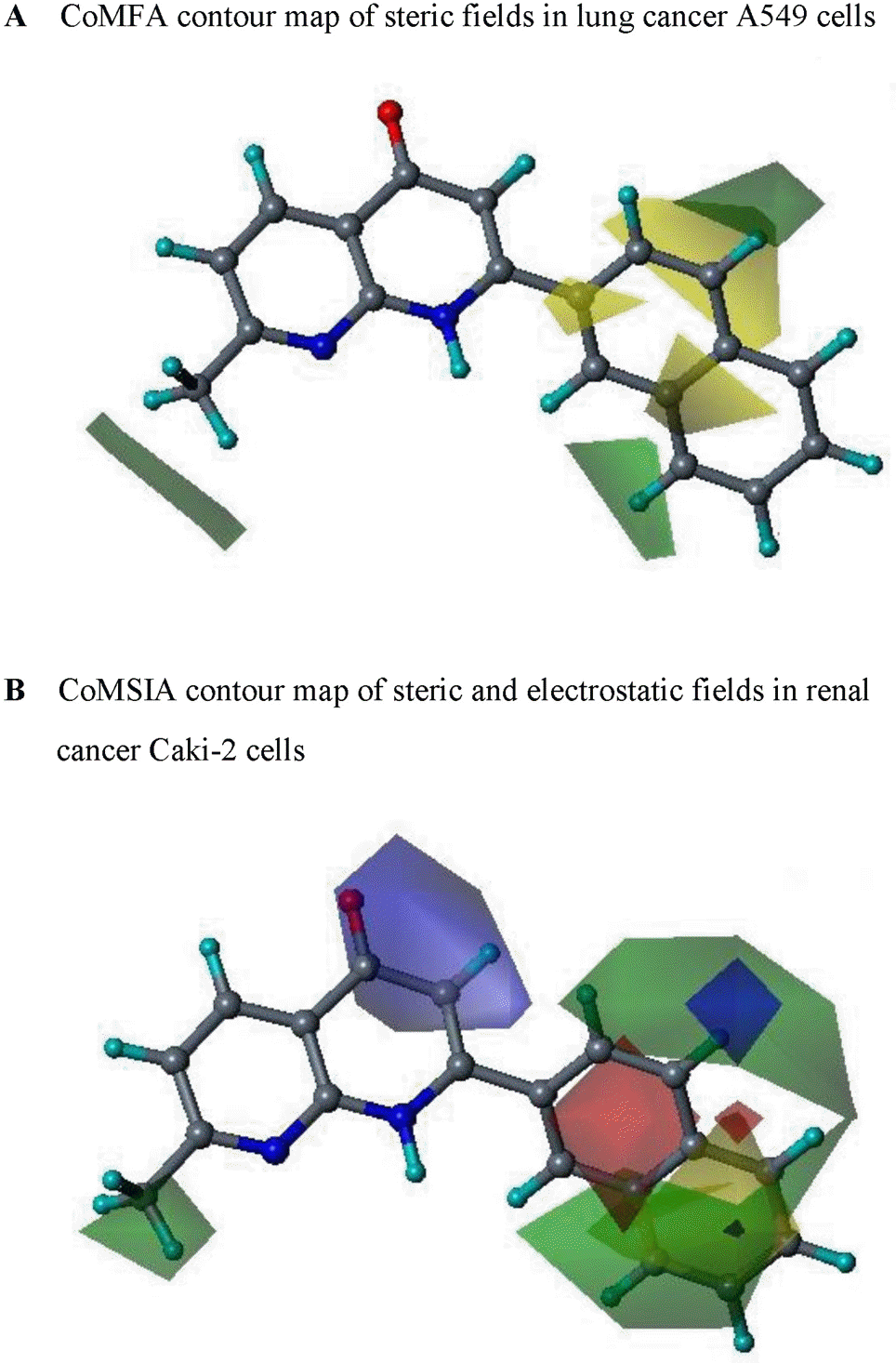
Table 2.
Statistical data of CoMFA and CoMSIA for two tumor cell lines
| Fielda | q2b | Nc | SEPd | r2ncve | SEEf | Fg | Contributionsa | |||
|---|---|---|---|---|---|---|---|---|---|---|
| S | E | D | A | |||||||
| Lung cancer A549 | ||||||||||
| CoMFA | ||||||||||
| S | 0.869 | 3 | 0.240 | 0.983 | 0.091 | 118.709 | 1 | |||
| E | 0.266 | 1 | 0.514 | 0.962 | 0.138 | 50.095 | 1 | |||
| SE | 0.820 | 3 | 0.281 | 0.983 | 0.092 | 115.575 | 0.843 | 0.157 | ||
| CoMSIA | ||||||||||
| S | 0.863 | 3 | 0.245 | 0.977 | 0.107 | 83.623 | 1 | |||
| E | 0.255 | 1 | 0.518 | 0.950 | 0.158 | 37.767 | 1 | |||
| D | 0.806 | 2 | 0.277 | 0.940 | 0.173 | 31.115 | 1 | |||
| A | 0.306 | 4 | 0.586 | 0.956 | 0.148 | 43.137 | 1 | |||
| SE | 0.577 | 4 | 0.457 | 0.977 | 0.108 | 83.536 | 0.491 | 0.509 | ||
| DA | 0.799 | 2 | 0.282 | 0.931 | 0.184 | 27.051 | 0.413 | 0.587 | ||
| Renal cancer Caki-2 | ||||||||||
| CoMFA | ||||||||||
| S | 0.412 | 1 | 0.338 | 0.989 | 0.054 | 184.305 | 1 | |||
| E | 0.838 | 4 | 0.208 | 0.984 | 0.064 | 126.912 | 1 | |||
| SE | 0.715 | 3 | 0.260 | 0.992 | 0.046 | 248.683 | 0.637 | 0.363 | ||
| CoMSIA | 1 | 0.372 | 0.976 | 0.080 | 81.450 | |||||
| S | 0.287 | 1 | ||||||||
| E | 0.827 | 3 | 0.202 | 0.985 | 0.062 | 135.235 | 1 | |||
| D | 0.538 | 2 | 0.314 | 0.946 | 0.120 | 35.053 | 1 | |||
| A | 0.485 | 4 | 0.371 | 0.948 | 0.118 | 36.104 | 1 | |||
| SE | 0.872 | 3 | 0.174 | 0.993 | 0.042 | 305.023 | 0.319 | 0.681 | ||
| DA | 0.619 | 3 | 0.301 | 0.916 | 0.150 | 21.837 | 0.323 | 0.677 | ||
Table 3.
Actual and predicted activity (pIC50) of training set




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download