Abstract
Glutamate-induced cobalt uptake reveals that non-NMDA glutamate receptors (GluRs) are present in rat taste bud cells. Previous studies involving glutamate induced cobalt staining suggest this uptake mainly occurs via kainate type GluRs. It is not known which of the 4 types of taste bud cells express subunits of kainate GluR. Circumvallate and foliate papillae of Sprague-Dawley rats (45∼60 days old) were used to search for the mRNAs of subunits of non-NMDA GluRs using RT-PCR with specific primers for GluR1–7, KA1 and KA2. We also performed RT-PCR for GluR5, KA1, PLCβ2, and NCAM/SNAP 25 in isolated single cells from taste buds. Taste epithelium, including circumvallate or foliate papilla, express mRNAs of GluR5 and KA1. However, non-taste tongue epithelium expresses no subunits of non-NMDA GluRs. Isolated single cell RT-PCR reveals that the mRNAs of GluR5 and KA1 are preferentially expressed in Type II and Type III cells over Type I cells.
References
Boughter Jr JD., Pumplin DW., Yu C., Christy RC., Smith DV. Differential expression of alpha-gustducin in taste bud populations of the rat and hamster. J Neurosci. 17:2852–2858. 1997.
Bowie D. External anions and cations distinguish between AMPA and kainate receptor gating mechanisms. J Physiol (London). 539:725–733. 2002.

Bradlley RM., King MS., Wang L., Shu X. Neurotransmitter and neuromodulator activity in the gustatory zone of the nucleus tractus solitarius. Chem Sens. 21:377–385. 1996.
Caicedo A., Kim KN., Roper SD. Glutamate-induced cobalt uptake reveals non-NMDA receptors in rat taste cells. J Comp Neurol. 417:315–324. 2000.

Castillo PE., Malenka RC., Nicoll RA. Kainate receptors mediate a slow postsynaptic current in hippocampal CA3 neurons. Nature. 388:182–186. 1997.

Chittajallu R., Vignes M., Dev KK., Barnes JM., Collingridge GL., Henley JM. Regulation of glutamate release by presynaptic kainate receptors in the hippocampus. Nature. 79:78–816. 1993.

Chung KM., Lee SB., Heur R., Cho YK., Lee CH., Jung HY., Chung SH., Lee SP., Kim KN. Glutamate-induced cobalt uptake elicited by kainate receptors in rat taste bud cells. Chem Senses. 30:137–143. 2005.

DeFazio RA., Dvoryanchikov G., Maruyama Y., Kim JW., Pereira E., Roper SD., Chaudhari N. Separate populations of receptor cells and presynaptic cells in mouse taste buds. J Neurosci. 26:3971–3980. 2006.

Dingledine R., Conn PJ. Peripheral Glutamate Receptors: molecular biology and role in taste sensation. J Nutr. 130:1039S–1042S. 2000.

Farbmann AI. Fine structure of taste bud. J Ultrastruct Res. 12:328–350. 1965.
Finger TE., Danilova V., Barrows J., Bartel DL., Vigers AJ., Stone L., Hellekant G., Kinnamon SC. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 310:1495–1499. 2005.

Herness MS., Chen Y. Serotonin inhibits calcium-activated K+ current in rat taste receptor cells. NeuroReport. 8:3527–3261. 1997.
Herness MS., Zhao FL., Lu SG., Kaya N., Shen T., Sun XD. Adrenergic signaling between rat taste recepter cells. J Physiol (Lond). 543:601–614. 2002.
Huang YJ., Maruyama Y., Lu KS., Pereira E., Plonsky I., Baur JE., Wu D., Roper SD. Mouse taste buds use serotonin as a neurotransmitter. J Neurosci. 25:843–847. 2005.

Jain S., Roper SD. Immunocytochemistry of GABA, glutamate, serotonin, and histamine in Necturus taste buds. J Comp Neurol. 307:675–682. 1991.
Kim KN., Caicedo A., Roper SD. Glutamate-induced cobalt uptake reveals non-NMDA receptors in developing rat taste buds. NeuroReport. 12:1715–1718. 2001.

Lee SB., Lee CH., Cho YK., Chung KM., Kim KN. Expression of kainate glutamate receptors in type II cells in taste buds of rats. Int J Oral Biol. 33:83–89. 2008.
Lerma J. Roles, and rules of kainate receptors in synaptic transmission. Nat Rev Neurosci. 4:481–495. 2003.

Madden DR. The structure and function of glutamate receptor ion channels. Nat Rev Neurosci. 3:91–101. 2002.

Mayer ML. Crystal structure of the GluR5 and GluR6 ligand binding cores: Molecular mechanisms underlying kainate receptor selectivity. Neuron. 45:539–552. 2005.
Mezei LM., Store DR. Purification of PCR products. Griffin HG, Griffin AM, editors. ed,. PCR technology: Current innovations. CRC Press;Boca Raton: p. p. 13–19. 1994.
Murray RG. Mammalian taste bud type III cell: a critical analysis. J Ultrastruct Mol Struct Res. 95:175–188. 1986.
Murray RG. The ultrastructure of taste buds. Friedmann I, editor. ed,. The ultrastructure of taste organs. Amsterdam, North Holland: p. p. 1–81. 1974.
Nagai T., Delay RJ., Welton J., Roper SD. Uptake and release of neurotransmitter candidates, [3H]serotonin, [3H]glutamate, and [3H]GABA, in taste buds of the mudpuppy. Nectrus maculosus. J Comp Neurol. 392:199–208. 1998.
Passafaro M., Nakagawa T., Sala C., Sheng M. GABAnergic neurotransmission in rat taste buds: immunocytochemical study for GABA and GABA transporter subtypes. Nature. 424:677–681. 2003.
Pumplin DW., Yu C., Smith DV. Light and dark cells of rat vallate taste buds are morphologically distinct cell types. J Comp Neurol. 378:380–410. 1997.

Raphael Y., Altschuler RA. Structure and innervation of the cochlea. Brain Res Bull. 60:397–422. 2003.

Roper SD. Cell communication in taste buds. Cell Mol Life Sci. 63:1494–1500. 2006.
Fig. 1.
Results of tissue RT-PCR of mRNA obtained from tongue epithelium and hippocampus. N, non-taste epithelium; F, epithelium including foliate papilla; CV, epithelium including circumvallate papilla; H, hippocampus as positive control; –, no tissue as negative control; M, size marker.
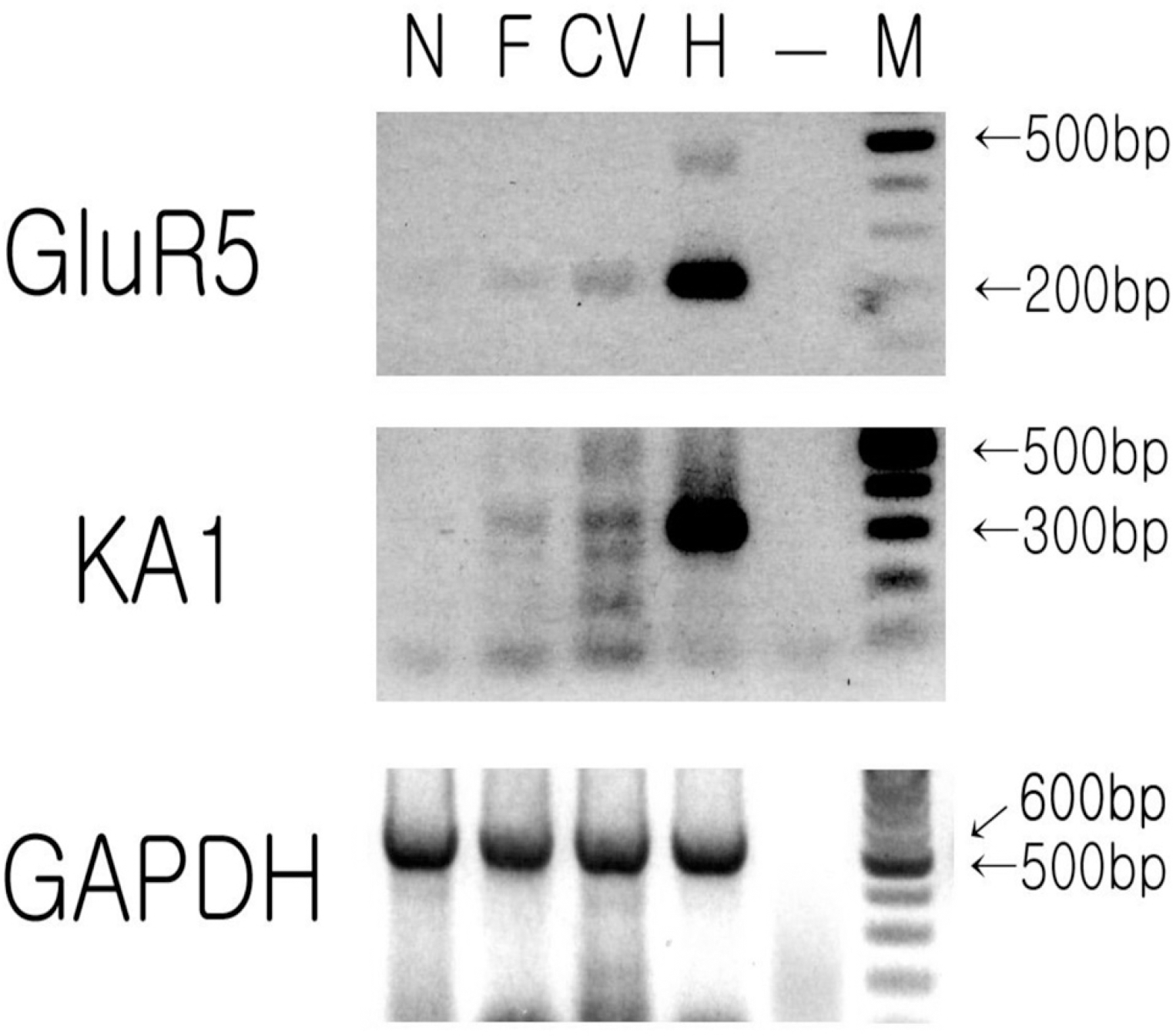
Fig. 2.
Representative isolated single cell RT-PCR results. Data is from 15 cells obtained from a single rat. Data from lanes 11 and 15 were discarded because both PLCβ2 and SNAP25 are expressed. Two cells express PLCβ2, another 2 cells express SNAP 25, 6 cell express GluR5, and 2 cells express KA1. Even cells in lanes 11 and 15 do not express KA1. However, GAPDH is expressed in all lanes except the negative control.
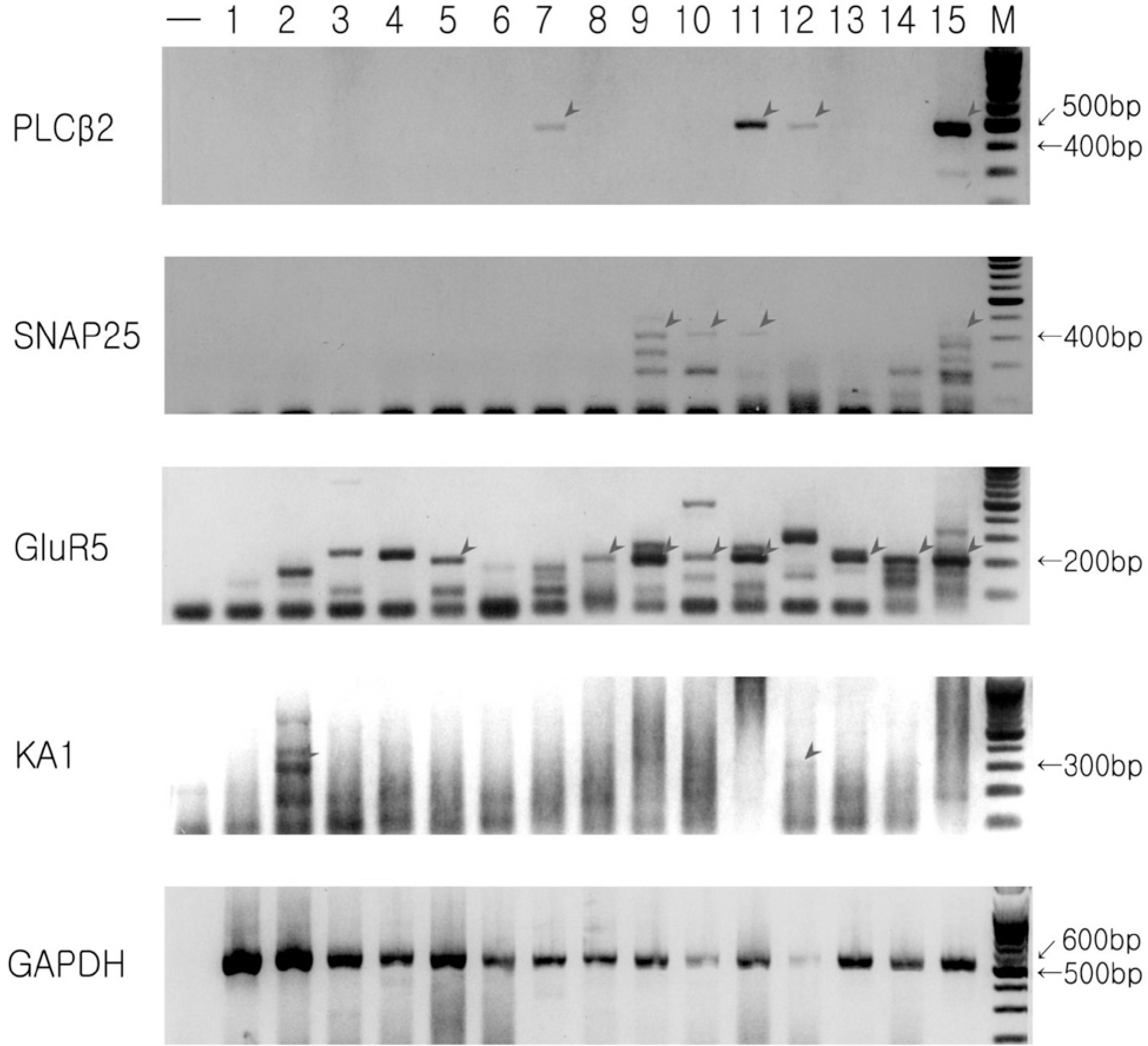
Fig. 3.
Results of isolated single cell RT-PCR for taste bud cells. Each column represents data from an individual taste cell. Each row indicates expression of different mRNAs.
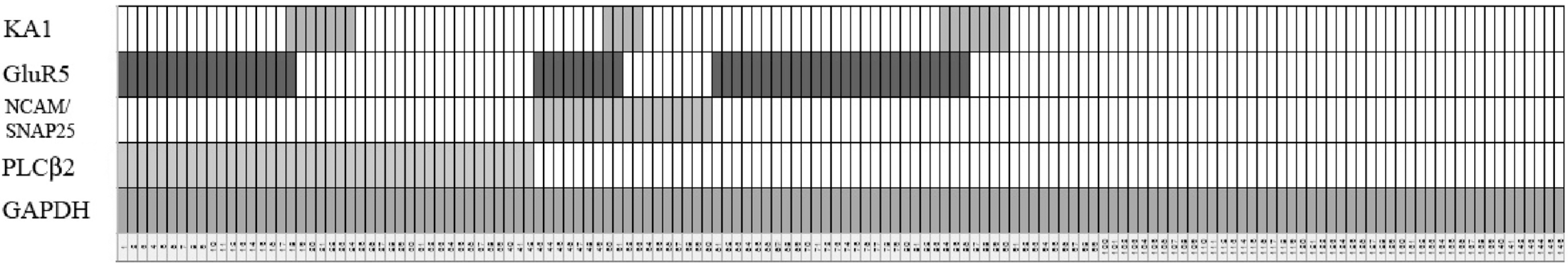
Table 1.
DNA sequences, annealing temperatures, and expected product sizes of specific primers of subunits of non-NMDA glutamate receptors used in RT-PCR
Table 2.
DNA sequences, annealing temperatures and expected product sizes for specific primers used in RT-PCR to test specific markers for cell type in taste buds (positive control GAPDH)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download