Abstract
Nitric oxide (NO), a diffusible gas, is produced in the central nervous system, including the spinal cord dorsal horn and the trigeminal nucleus, the first central areas processing nociceptive information from periphery. In the spinal cord, it has been demonstrated that NO acts as pronociceptive or antinociceptive mediators, apparently in a concentration-dependent manner. However, the central role of NO in the trigeminal nucleus remains uncertain in support of processing the orofacial nociception. Thus, we here investigated the central role of NO in formalin (3%)-induced orofacial pain in rats by administering membrane-permeable or -impermeable inhibitors, relating to the NO signaling pathways, into intracisternal space. The intracisternal pretreatments with the NO synthase inhibitor L-NAME, the NO-sensitive guanylate cyclase inhibitor ODQ, and the protein kinase C inhibitor GF109203X, all of which are permeable to the cell membrane, significantly reduced the formalin-induced pain, whereas the membrane-impermeable NO scavenger PTIO significantly enhanced it, compared to vehicle controls. These data suggest that an overall effect of NO production in the trigeminal nucleus is pronociceptive, but NO extracellularly diffused out of its producing neurons would have an antinociceptive action.
References
Bredt DS., Ferris CD., Snyder SH. Nitric oxide synthase regulatory sites. Phosphorylation by cyclic AMP-dependent protein kinase, protein kinase C, and calcium/calmodulin protein kinase; identification of flavin and calmodulin binding sites. J Biol Chem. 267:10976–10981. 1992.

Clavelou P., Dallel R., Orliaguet T., Woda A., Raboisson P. The orofacial formalin test in rats: effects of different formalin concentrations. Pain. 62:295–301. 1995.

Dinerman JL., Steiner JP., Dawson TM., Dawson V., Snyder SH. Cyclic nucleotide dependent phosphorylation of neuronal nitric oxide synthase inhibits catalytic activity. Neuropharmacology. 33:1245–1251. 1994.

Ding JD., Weinberg RJ. Localization of soluble guanylyl cyclase in the superficial dorsal horn. J Comp Neurol. 495:668–678. 2006.

Duarte ID., Ferreira SH. L-NAME causes antinociception by stimulation of the arginine-NO-cGMP pathway. Mediators Inflamm. 9:25–30. 2000.

Gerber G., Kangrga I., Ryu PD., Larew JS., Randic M. Multiple effects of phorbol esters in the rat spinal dorsal horn. J Neurosci. 9:3606–3617. 1989.

Hama AT., Sagen J. Induction of spinal NADPH-diaphorase by nerve injury is attenuated by adrenal medullary transplants. Brain Res. 640:345–351. 1994.

Herdegen T., Rudiger S., Mayer B., Bravo R., Zimmermann M. Expression of nitric oxide synthase and colocalisation with Jun, Fos and Krox transcription factors in spinal cord neurons following noxious stimulation of the rat hindpaw. Brain Res Mol Brain Res. 22:245–258. 1994.

Knowles RG., Palacios M., Palmer RM., Moncada S. Formation of nitric oxide from L-arginine in the central nervous system: a transduction mechanism for stimulation of the soluble guanylate cyclase. Proc Natl Acad Sci U S A. 86:5159–5162. 1989.

Lin Q., Peng YB., Willis WD. Possible role of protein kinase C in the sensitization of primate spinothalamic tract neurons. J Neurosci. 16:3026–3034. 1996.

Meller ST., Gebhart GF. Nitric oxide (NO) and nociceptive processing in the spinal cord. Pain. 52:127–136. 1993.

Nakane M., Mitchell J., Forstermann U., Murad F. Phosphorylation by calcium calmodulin-dependent protein kinase II and protein kinase C modulates the activity of nitric oxide synthase. Biochem Biophys Res Commun. 180:1396–1402. 1991.

Palecek J., Paleckova V., Willis WD. The effect of phorbol esters on spinal cord amino acid concentrations and responsiveness of rats to mechanical and thermal stimuli. Pain. 80:597–605. 1999.

Ruscheweyh R., Goralczyk A., Wunderbaldinger G., Schober A., Sandkuhler J. Possible sources and sites of action of the nitric oxide involved in synaptic plasticity at spinal lamina I projection neurons. Neuroscience. 141:977–988. 2006.

Schmidtko A., Gao W., Konig P., Heine S., Motterlini R., Ruth P., Schlossmann J., Koesling D., Niederberger E., Tegeder I., Friebe A., Geisslinger G. cGMP produced by NO-sensitive guanylyl cyclase essentially contributes to inflammatory and neuropathic pain by using targets different from cGMP-dependent protein kinase I. J Neurosci. 28:8568–8576. 2008.

Schmidtko A., Tegeder I., Geisslinger G. No NO, no pain? The role of nitric oxide and cGMP in spinal pain processing. Trends Neurosci. 32:339–346. 2009.

Semos ML., Headley PM. The role of nitric oxide in spinal nociceptive reflexes in rats with neurogenic and non-neurogenic peripheral inflammation. Neuropharmacology. 33:1487–1497. 1994.

Sluka KA., Willis WD. The effects of G-protein and protein kinase inhibitors on the behavioral responses of rats to intradermal injection of capsaicin. Pain. 71:165–178. 1997.

Sousa AM., Prado WA. The dual effect of a nitric oxide donor in nociception. Brain Res. 897:9–19. 2001.

Tegeder I., Schmidtko A., Niederberger E., Ruth P., Geisslinger G. Dual effects of spinally delivered 8-bromo-cyclic guanosine monophosphate (8-bromo-cGMP) in formalin-induced nociception in rats. Neurosci Lett. 332:146–150. 2002.

Valtschanoff JG., Weinberg RJ., Rustioni A., Schmidt HH. Nitric oxide synthase and GABA colocalize in lamina II of rat spinal cord. Neurosci Lett. 148:6–10. 1992.

Yashpal K., Pitcher GM., Parent A., Quirion R., Coderre TJ. Noxious thermal and chemical stimulation induce increases in 3H-phorbol 12,13-dibutyrate binding in spinal cord dorsal horn as well as persistent pain and hyperalgesia, which is reduced by inhibition of protein kinase C. J Neurosci. 15:3263–3272. 1995.

Yeo JF. Does nitric oxide play a role in orofacial pain transmission? Ann N Y Acad Sci. 962:151–160. 2002.

Yeo JF., Tang FR., Leong SK. Ultrastructural study of NADPH-d positive neurons in laminae I and II of the rat caudal spinal trigeminal nucleus. Int J Neurosci. 91:29–43. 1997.
Fig. 1.
Opposite effects of central NOS inhibition and NO scavenging on formalin-induced orofacial pain. Formalin-induced characteristic rubbing behaviors were reduced by intracisternal injection of L-NAME, a NOS inhibitor, in the second phase (10∼60 min), while enhanced by that of PTIO, a scavenger of NO, both in the first (0∼6 min) and the second phases (A). A histogram (B), summarizing the first and the second phases of the formalin-induced pain behaviors observed in rats, demonstrates the significant reduction in the second phase by L-NAME (n=4) and the significant enhancement both in the first and the second phases by PTIO (n=5), compared to saline (n=4). Formalin was injected into the right upper lip at time zero, and the number of rubbing behaviors was counted. ∗∗p<0.01 and ∗p<0.05 vs. saline, #p<0.05 and ##p<0.01 vs. L-NAME.
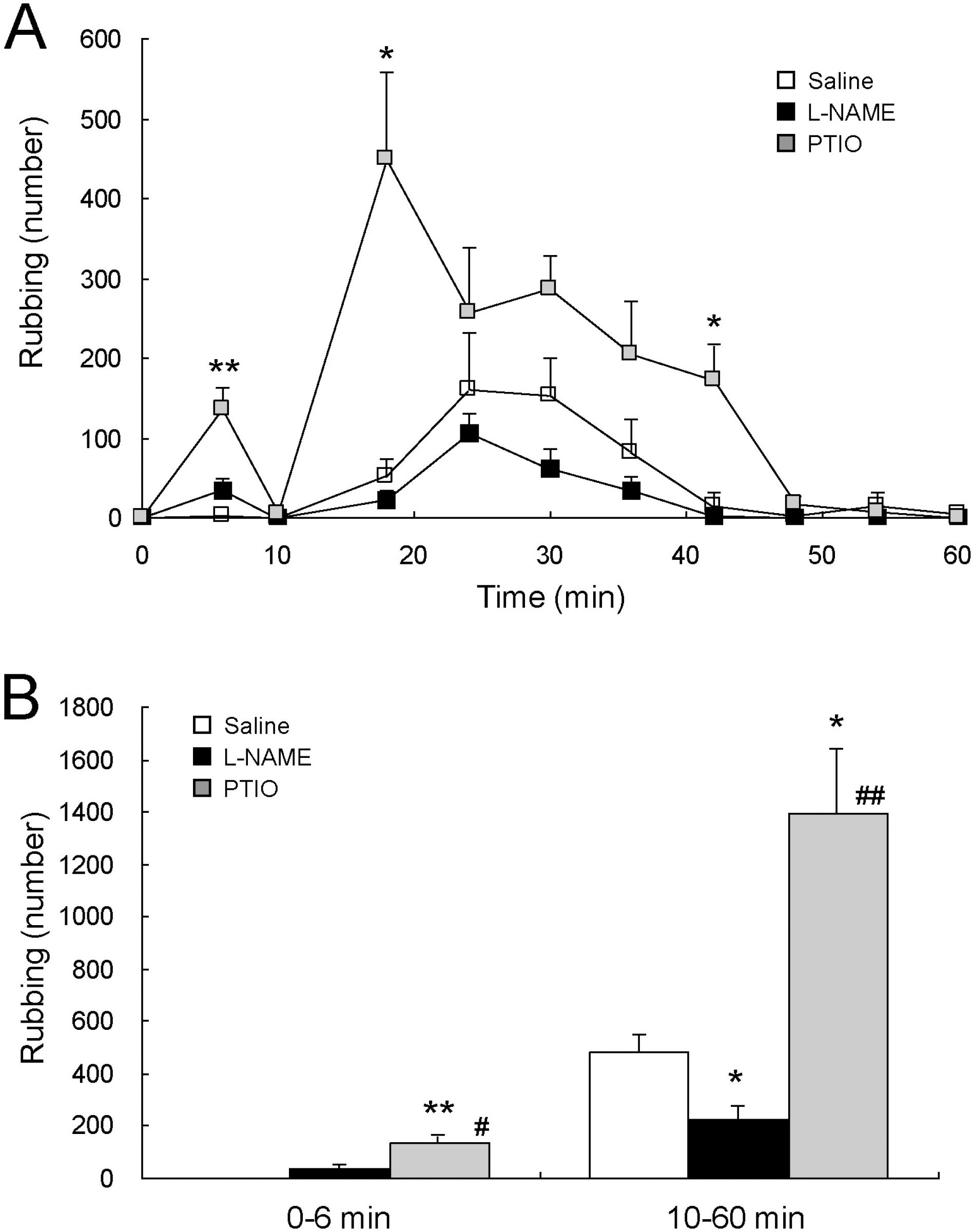
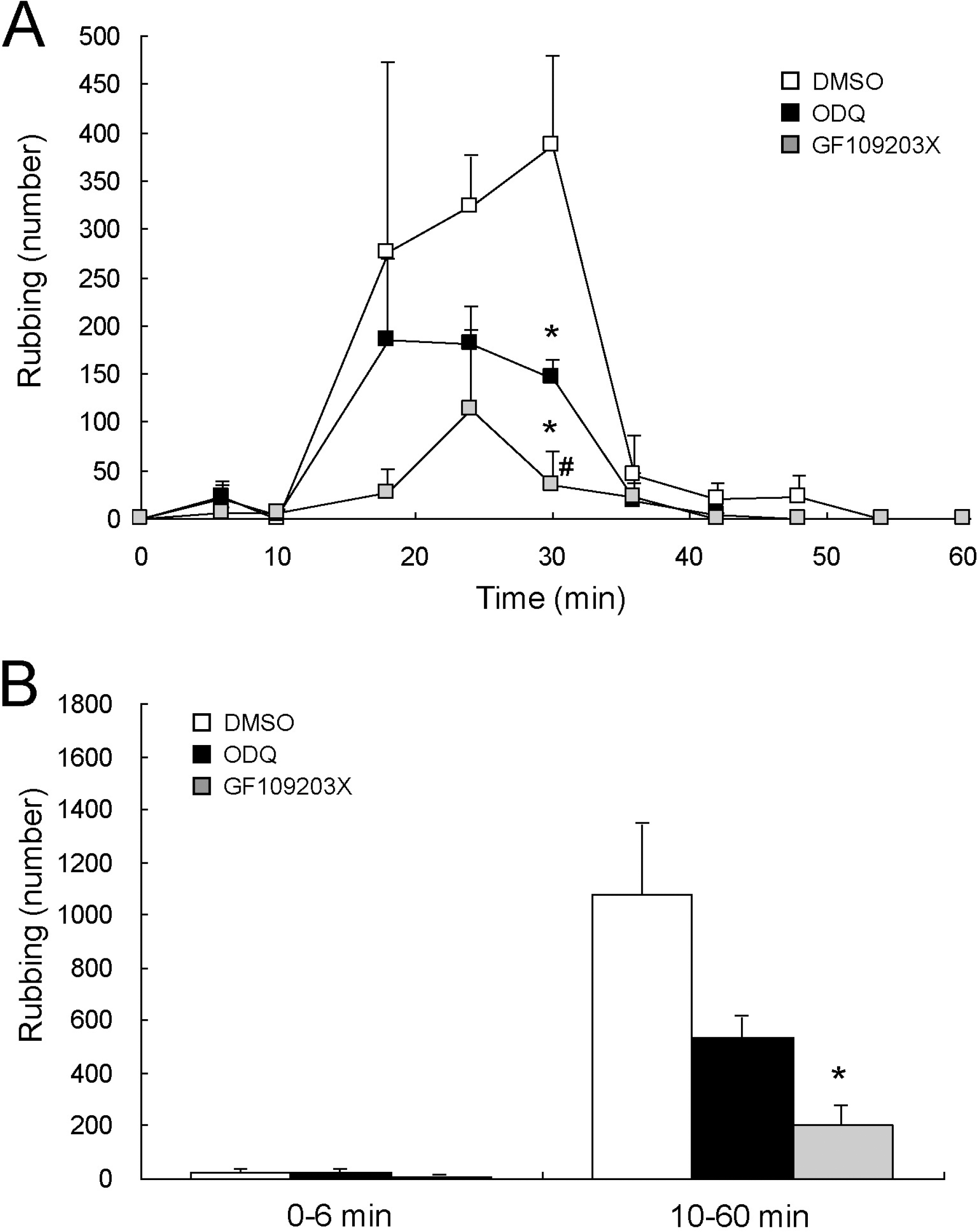
Fig. 2.
Reduction of formalin-induced orofacial pain by central inhibition of NO-sensitive guanylate cyclase. Formalin-induced characteristic rubbing behaviors were reduced in the second phase by intracisternal injection of ODQ, an inhibitor of NO-sensitive guanylate cyclase (A). GF109203X, a PKC inhibitor, used as a positive effect control, also reduced the second phase of formalin-induced orofacial pain behavior. A histogram (B), summarizing the first and the second phases of the formalin-induced pain behaviors observed in rats, demonstrates the tendency of reduction by ODQ (n=5) and the significant reduction by GF109203X (n=3) in the second phase, compared to that of DMSO negative control (n=3). Formalin was injected into the right upper lip at time zero, and the number of rubbing behaviors was counted. ∗p<0.05 vs. DMSO, #p<0.05 vs. ODQ.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download