Abstract
Spontaneous hypertensive rats (SHR) are an established model of genetic hypertension. Vascular smooth muscle cells (VSMC) from SHR proliferate faster than those of control rats (Wistar-Kyoto rats; WKY). We tested the hypothesis that induction of heme oxygenase (HO)-1 induced by aprotinin inhibits VSMC proliferation through cell cycle arrest in hypertensive rats. Aprotinin treatment inhibited VSMC proliferation in SHR more than in normotensive rats. These inhibitory effects were associated with cell cycle arrest in the G1 phase. Tin protoporphyrin IX (SnPPIX) reversed the anti-proliferative effect of aprotinin in VSMC from SHR. The level of cyclin D was higher in VSMC of SHR than those of WKY. Aprotinin treatment downregulated the cell cycle regulator, cyclin D, but upregulated the cyclin-dependent kinase inhibitor, p21, in VSMC of SHR. Aprotinin induced HO-1 in VSMC of SHR, but not in those of control rats. Furthermore, aprotinin-induced HO-1 inhibited VSMC proliferation of SHR. Consistently, VSMC proliferation in SHR was significantly inhibited by transfection with the HO-1 gene. These results indicate that induction of HO-1 by aprotinin inhibits VSMC proliferation through cell cycle arrest in hypertensive rats.
REFERENCES
Bidstrup BP., Royston D., Sapsford RN., Taylor KM. Reduction in blood loss and blood use after cardiopulmonary bypass with high dose aprotinin (Trasylol). J Thorac Cardiovasc Surg. 97:364–372. 1989.

Chang T., Wu L., Wang R. Inhibition of vascular smooth muscle cell proliferation by chronic hemin treatment. Am J Physiol Heart Circ Physiol. 295:H999–H1007. 2008.

Dong Y., Chi SL., Borowsky AD., Fan Y., Weiss RH. Cytosolic p21Waf1/Cip1 increases cell cycle transit in vascular smooth muscle cells. Cell Signal. 16:263–269. 2004.

Duckers HJ., Boehm M., True ., Yet SF., San H., Park JL., Clinton Webb R., Lee ME., Nabel GJ., Nabel EG. Heme oxygenase-1 protects against vascular constriction and proliferation. Nat Med. 7:693–698. 2001.

Dulic V., Lees E., Reed SI. Association of human cyclin E with a periodic G1-S phase protein kinase. Science. 257:1958–1961. 1992.

Englberger L., Kipfer B., Berdat PA., Nydegger UE., Carrel TP. Aprotinin in coronary operation with cardiopulmonary bypass: does “low-dose” aprotinin inhibit the inflammatory response? Ann Thorac Surg. 73:1897–1904. 2002.

Intengan HD., Schiffrin EL. Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension. 38:581–587. 2001.
Jeon EM., Choi HC., Lee KY., Chang KC., Kang YJ. Hemin inhibits hypertensive rat vascular smooth muscle cell proliferation through regulation of cyclin D and p21. Arch Pharm Res. 32:375–382. 2009.

Lee DH., Choi HC., Lee KY., Kang YJ. Aprotinin inhibits vascular smooth muscle cell inflammation and proliferation via induction of HO-1. Korean J Physiol Pharmacol. 13:123–130. 2009.

Levy JH. Pharmacologic preservation of the hemostatic system during cardiac surgery. Ann Thorac Surg. 72:S1814–1820. 2001.

Mangano DT., Tudor IC., Dietzel C. The risk associated with aprotinin in cardiac surgery. N Engl J Med. 354:353–365. 2006.

McEvoy MD., Taylor AG., Zavadzkas JA., Mains IM., Ford RL., Stroud RE., Jeffords LB., Beck CU., Reeves ST., Spinale FG. Aprotinin exerts differential and dose-dependent effects on myocardial contractility, oxidative stress, and cytokine release after ischemia-reperfusion. Ann Thorac Surg. 86:568–575. 2008.

Ndisang JF., Wu L., Zhao W., Wang R. Induction of heme oxygenase-1 and stimulation of cGMP production by hemin in aortic tissues from hypertensive rats. Blood. 101:3893–3900. 2003.

Ndisang JF., Zhao W., Wang R. Selective regulation of blood pressure by heme oxygenase-1 in hypertension. Hypertension. 40:315–321. 2002.

Ngaage DL., Cale AR., Cowen ME., Griffin S., Guvendik L. Aprotinin in primary cardiac surgery: operative outcome of propensity score-matched study. Ann Thorac Surg. 86:1195–1202. 2008.

Peyton KJ., Reyna SV., Chapman GB., Ensenat D., Liu XM., Wang H., Schafer AI., Durante W. Heme oxygenase-1-derived carbon monoxide is an autocrine inhibitor of vascular smooth muscle cell growth. Blood. 99:4443–4448. 2002.

Resink TJ., Scott-Burden T., Baur U., Bühler FR. Increased proliferation fate and phosphoinositide turnover in cultured smooth muscle cells from spontaneously hypertensive rats. J Hypertens Suppl. 5:S145–148. 1987.
Taillé C., Almolki A., Benhamed M., Zedda C., Mégret J., Berger P., Leséche G., Fadel E., Yamaguchi T., Marthan R., Aubier M., Boczkowski J. Heme oxygenase inhibits human airway smooth muscle proliferation via a bilirubin-dependent modulation of ERK1/2 phosphorylation. J Biol Chem. 278:27160–27168. 2003.

Tanner FC., Greutert H., Barandier C., Frischknecht K., Lüscher TF. Different cell cycle regulation of vascular smooth muscle in genetic hypertension. Hypertension. 42:184–188. 2003.

Fig. 1.
Aprotinin treatment inhibits VSMC proliferation of SHR. VSMC were treated with aprotinin (1~10 μM) for 24 h, and cell proliferation determined by MTT assay (A). Bars represent the mean±SEM of four different experiments. ∗p<0.01 compared with WKY control, †p<0.01 compared with SHR control. Cell were treated with or without 10 μM and cell numbers determined after 0, 1, 2 and 3 days by cell counts (B). Bars represent the mean±SEM of three independent experiments.
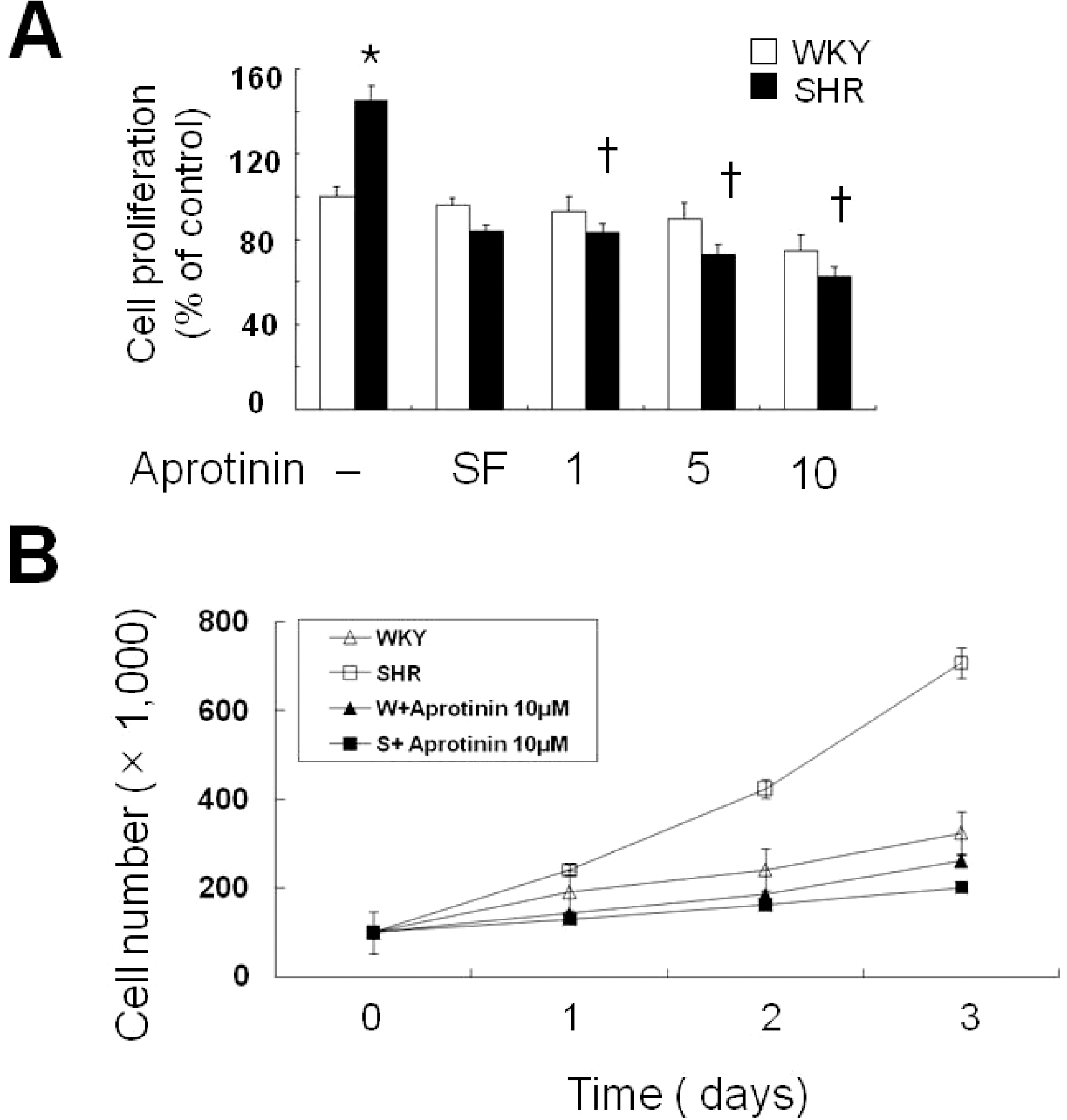
Fig. 2.
Aprotinin treatment inhibits cell cycle progression of VSMC from SHR through HO-1. VSMC were treated with 10 μM aprotinin or 10 μM aprotinin plus 1 μM SnppIX for 24 h, and then analyzed for DNA content by flow cytometry. The percentages of cells in G1 phase are graphically represented (A). The data are representative of three separate experiments (B). ∗p<0.01 compared with WKY control, †P<0.001 compared with SHR control, ¶p<0.001 compared with 10 μM aprotinin-treated SHR. Bars represents the mean±SEM of four independent experiments.
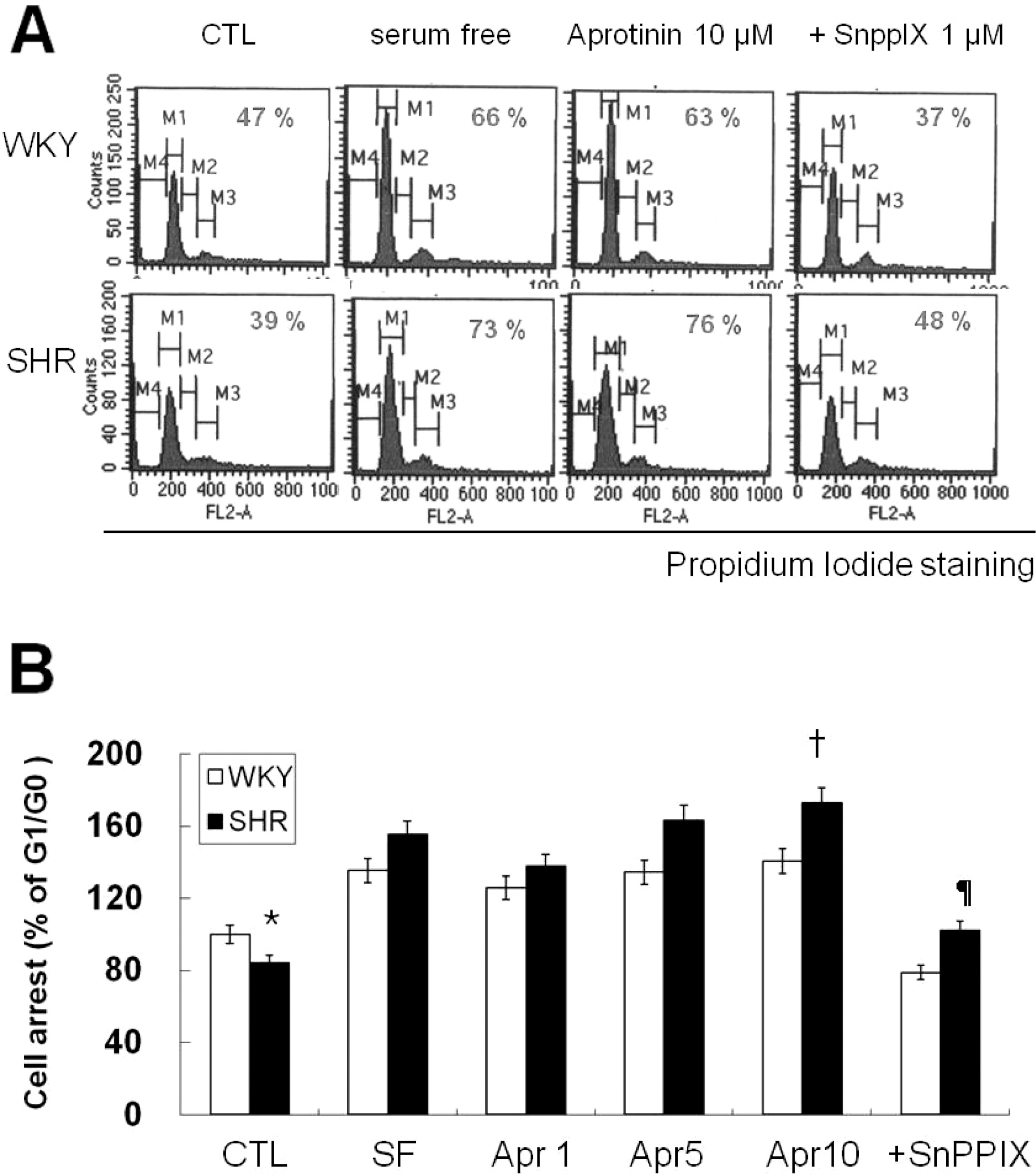
Fig. 3.
Aprotinin increases HO-1 and p21 but decreases cyclin D levels. Quiescent VSMC were stimulated with 10% FBS then serum starved for the indicated time, and cell lysates were processed for the detection of cyclin D, CDK4, and p21 by Western blot (A). Cells were treated with aprotinin (1~10 μM) for 48 h, and cell lysates were processed for the detection of HO-1, cyclin D, and p21 by western blot (B). The blots are representative of three independent experiments.
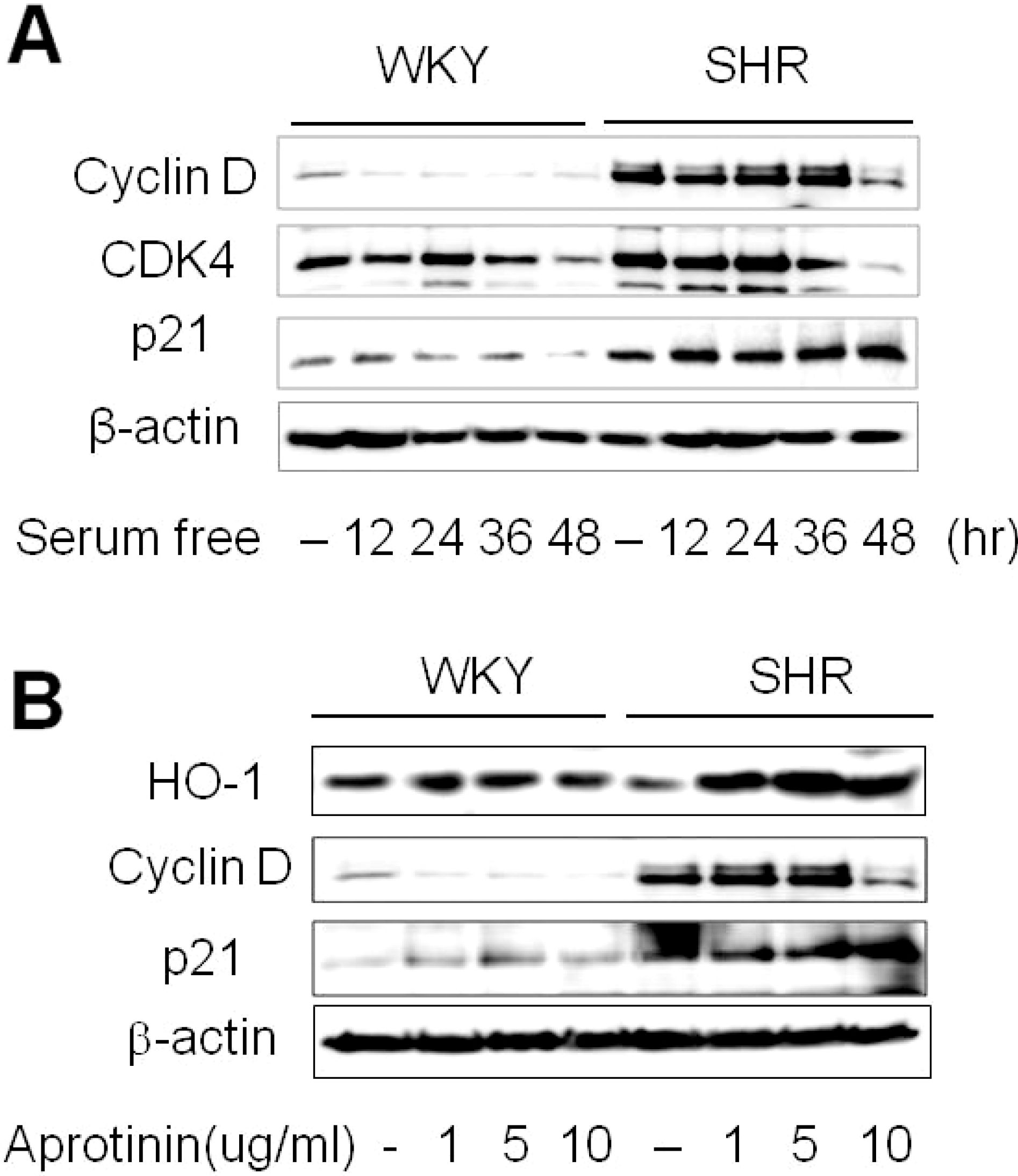
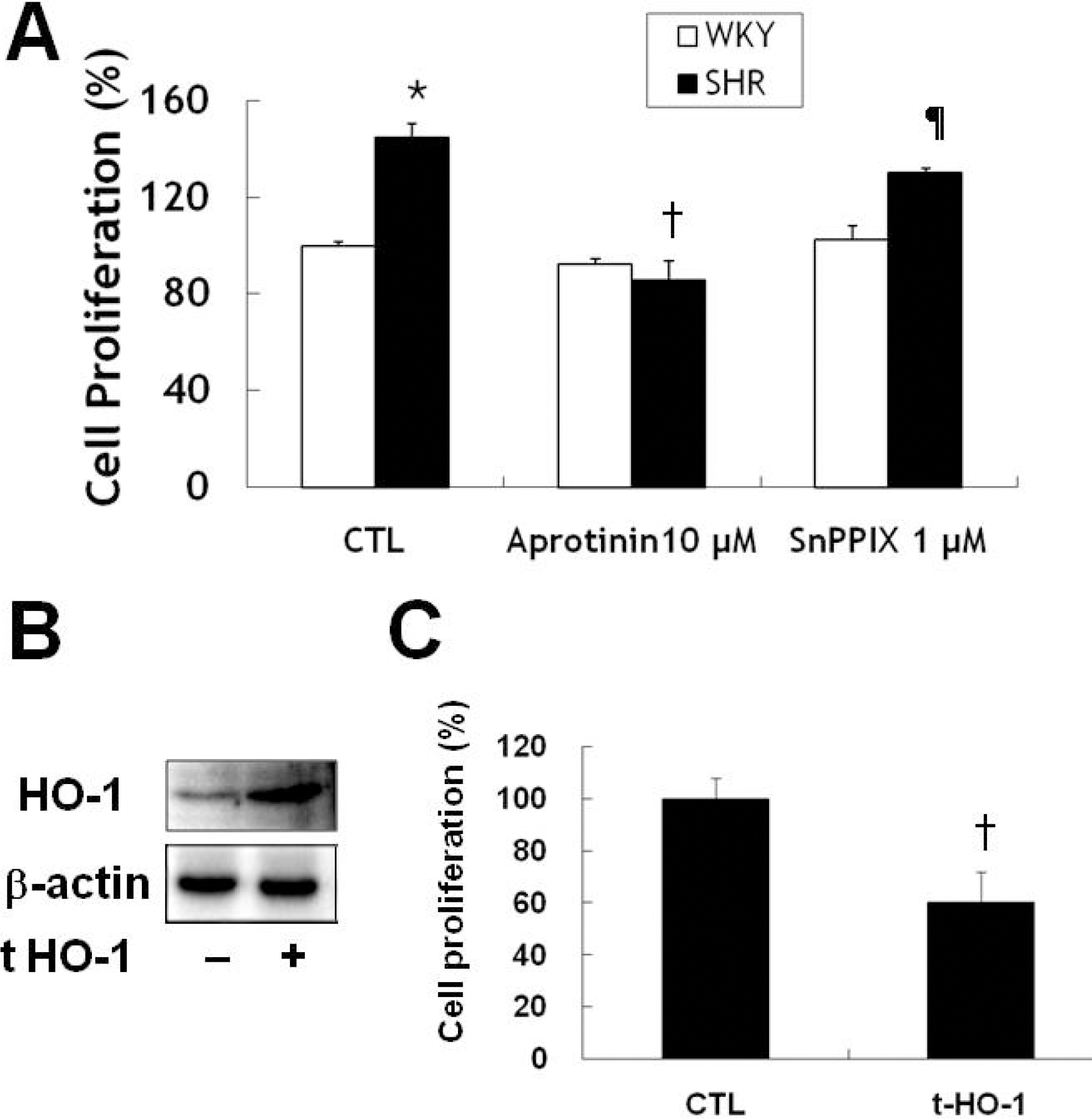
Fig. 4.
Aprotinin treatment inhibits VSMC proliferation of SHR through HO-1. VSMC were treated with 10 μM aprotinin or 10 μM aprotinin plus 1 μM SnppIX for 24 h, and cell proliferation determined by MTT assay (A). ∗p<0.01 compared with WKY control, †p<0.001 compared with SHR control, ¶p<0.001 compared with 10 μM aprotinin-treated SHR. Cells were transfected with mouse HO-1 DNA for 3 h and cell lysates were processed for the detection of HO-1 by Western blot (B). The blots are representative of three individual experiments. Cells were transfected with mouse HO-1 DNA for 3 h, incubated with 10% FBS for 24 h, and then cell proliferation was determined by MTT assay (C). †p<0.001 compared with SHR control. Bars represent the mean±SEM of four independent experiments.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download