Abstract
The dried roots of Danshen (Salvia miltiorrhiza) and Sanchi (Panax notoginseng) have been widely used in traditional Chinese medicine for promoting blood circulation as well as various other bodily functions. Here we investigated the effects of a mixture of aqueous extracts of Danshen and Sanchi, named PASEL, on blood pressure and vascular contractility in rats. Orally administered PASEL (62.5 mg/kg and 250 mg/kg, for 5 weeks) lowered the blood pressure of spontaneous hypertensive rats (SHR) but this was not observed in normal Wistar-Kyoto rats (WKR). We then investigated the effects of PASEL on the arterial contraction of the small branches of cerebral arteries (CAs) and large conduit femoral arteries (FAs) in rats. PASEL did not affect high-K (KCl 60 mM)- or phenyleprine (PhE)-induced contracture of FAs. The myogenic response, a reactive arterial constriction in response to increased luminal pressure, of small CA was dose-dependently suppressed by PASEL in SHR as well as control rats. Interestingly, the KCl-induced contraction of small CAs was slowly reversed by PASEL, and this effect was more prominent in SHR than control WKR. PASEL did not inhibit angiotensin-converting enzyme (ACE) activity. These results demonstrated that the antihypertensive effect of PASEL might be primarily mediated by altering the arterial MR, not by direct inhibition of L-type Ca2+ channels or by ACE inhibition.
REFERENCES
Ahn DS., Choi SK., Kim YH., Cho YE., Shin HM., Morgan KG., Lee YH. Enhanced stretch-induced myogenic tone in the basilar artery of spontaneously hypertensive rats. J Vasc Res. 44:182–191. 2007.

Chan P., Thomas GN., Tomlinson B. Protective effects of trilinolein extracted from Panax notoginseng against cardiovascular disease. Acta Pharmacol Sin. 23:1157–1162. 2002.
Chuang CM., Hsieh CL., Lin HY., Lin JG. Panax Notoginseng Burk attenuates impairment of learning and memory functions and increases ED1, BDNF and beta-secretase immunoreactive cells in chronic stage ischemia-reperfusion injured rats. Am J Chin Med. 36:685–693. 2008.
Dora KA. Does arterial myogenic tone determine blood flow distribution in vivo? Am J Physiol Heart Circ Physiol. 289:H1323–H1325. 2005.
Feihl F., Liaudet L., Levy BI., Waeber B. Hypertension and microvascular remodelling. Cardiovas Res. 78:274–285. 2008.

Gebremedhin D., Lange AR., Lowry TF., Reza Taheri MR., Birks EK., Hudetz AG., Narayanan J., Falck JR., Okamoto H., Roman RJ., Nithipatikom K., Campbell WB., Harder DR. Production of 20-HETE and its role in autoregulation of cerebral blood flow. Circ Res. 87:60–65. 2000.

Guan YY., Zhou JG., Zhang Z., Wang GL., Cai BX., Hong L., Qiu QY., He H. Ginsenoside-Rd from panax notoginseng blocks Ca2+ influx through receptor- and store-operated Ca2+ channels in vascular smooth muscle cells. Eur J Pharmacol. 548:129–136. 2006.
Hill MA., Davis MJ., Meininger GA., Potocnik SJ., Murphy TV. Arteriolar myogenic signalling mechanisms: Implications for local vascular function. Clin Hemorheol Microcirc. 34:67–79. 2006.
Jarajapu YP., Knot HJ. Relative contribution of Rho kinase and protein kinase C to myogenic tone in rat cerebral arteries in hypertension. Am J Physiol Heart Circ Physiol. 289:H1917–H1922. 2005.

Kang DG., Oh H., Chung HT., Lee HS. Inhibition of angiotensin converting enzyme by lithospermic acid B isolated from radix Salviae miltiorrhiza Bunge. Phytother Res. 17:917–920. 2003.
Kang DG., Yun YG., Ryoo JH., Lee HS. Anti-hypertensive effect of water extract of danshen on renovascular hypertension through inhibition of the renin angiotensin system. Am J Chin Med. 30:87–93. 2002.

Keller M., Lidington D., Vogel L., Peter BF., Sohn HY., Pagano PJ., Pitson S., Spiegel S., Pohl U., Bolz SS. Sphingosine kinase functionally links elevated transmural pressure and increased reactive oxygen species formation in resistance arteries. FASEB J. 20:702–704. 2006.

Koller A. Signaling pathways of mechanotransduction in arteriolar endothelium and smooth muscle cells in hypertension. Microcirculation. 9:277–294. 2002.

Lam FF., Yeung JH., Cheung JHY., Or PM. Pharmacological evidence for calcium channel inhibition by Danshen (Salvia miltiorrhiza) on rat isolated femoral artery. J Cardiovasc Pharmacol. 47:13–145. 2006a.

Lam FFY., Seto SW., Kwan YW., Yeung JH., Chan P. Activation of the iberiotoxin-sensitive BKCa channels by salvianolic acid B of the porcine coronary artery smooth muscle cells. Eur J Pharmacol. 546:28–35. 2006b.

Li H., Deng CQ., Chen BY., Zhang SP., Liang Y., Luo XG. Total saponins of Panax Notoginseng modulate the expression of caspases and attenuate apoptosis in rats following focal cerebral ischemia-reperfusion. J Ethnopharmacol. 121:412–418. 2009.

Mederos Y Schnitzler M., Storch U., Meibers S., Nurwakagari P., Breit A., Essin K., Gollasch M., Gudermann T. G(q)-coupled receptors as mechanosensors mediating myogenic vasoconstriction. EMBO J. 27:3092–3103. 2008.

Schubert R., Lidington D., Bolz SS. The emerging role of Ca2+ sensitivity regulation in promoting myogenic vasoconstriction. Cardiovasc Res. 77:8–18. 2008.
Sun K., Wang CS., Guo J., Horie Y., Fang SP., Wang F., Liu YY., Liu LY., Yang JY., Fan JY., Han JY. Protective effects of ginsenoside Rb1, ginsenoside Rg1, and notoginsenoside R1 on lipopolysaccharide-induced microcirculatory disturbance in rat mesentery. Life Sci. 81:509–518. 2007.

Fig. 1.
Effects of PASEL treatment on blood pressure (BP) of SHR and control rats. (A) BP measurements were conducted using tail-cuff methods in WKY control and SHR (n=5, respectively) for five weeks. PASEL was applied daily (62.5 and 250 mg/kg, p.o.) for five weeks. The BP of SHR was lowered from the first week of PASEL application, while not completely normalized to the control level. The mean BP values are indicated in Fig. (B) The BP of each animal was normalized (100%) to the initial level and the mean values are summarized. (C, D) Summary of the heart rates (beat per minute, BPM) and body weights (g).
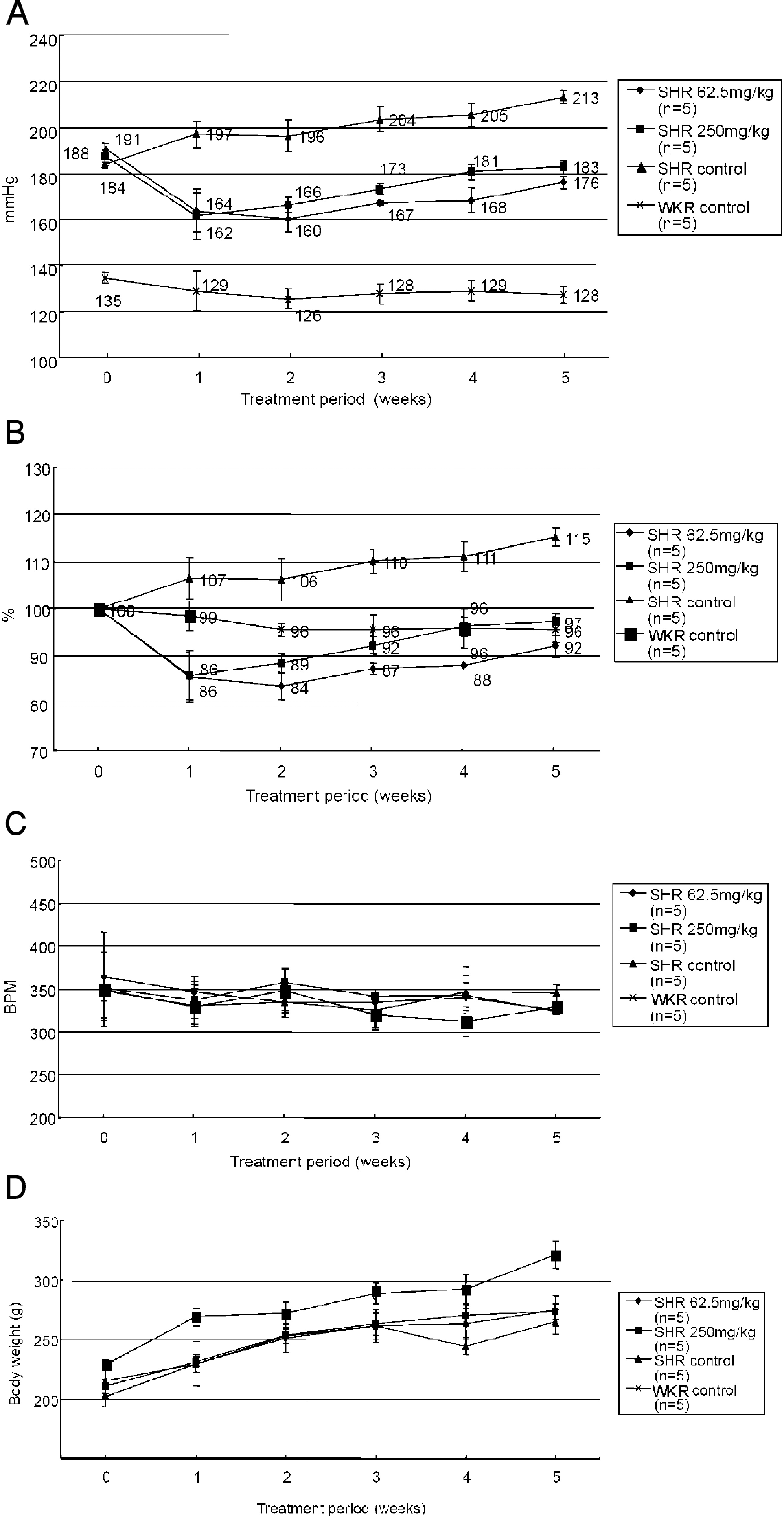
Fig. 2.
No significant effect of PASEL on high K+- and phenyleprine-induced contraction of large femoral artery. (A, B) Representative traces of isometric contraction of femoral arteries induced by 60 mM KCl (60 K) or 5 μM of phenyleprine (PhE). After confirming the consistent contractile responses to repetitive application of KCl or PhE (left panels), PASEL was applied (right panels). Neither 60 K- nor PhE-induced contraction was affected by pretreatment of the animals with 100 μg/ml PASEL. (C) Summary of the contractile responses in the absence (open bars) or the presence of PASEL (100 μg/ml, closed bars). Contractile responses were normalized (%) to the initial control amplitude. The number of tested arteries is indicated above each bar.
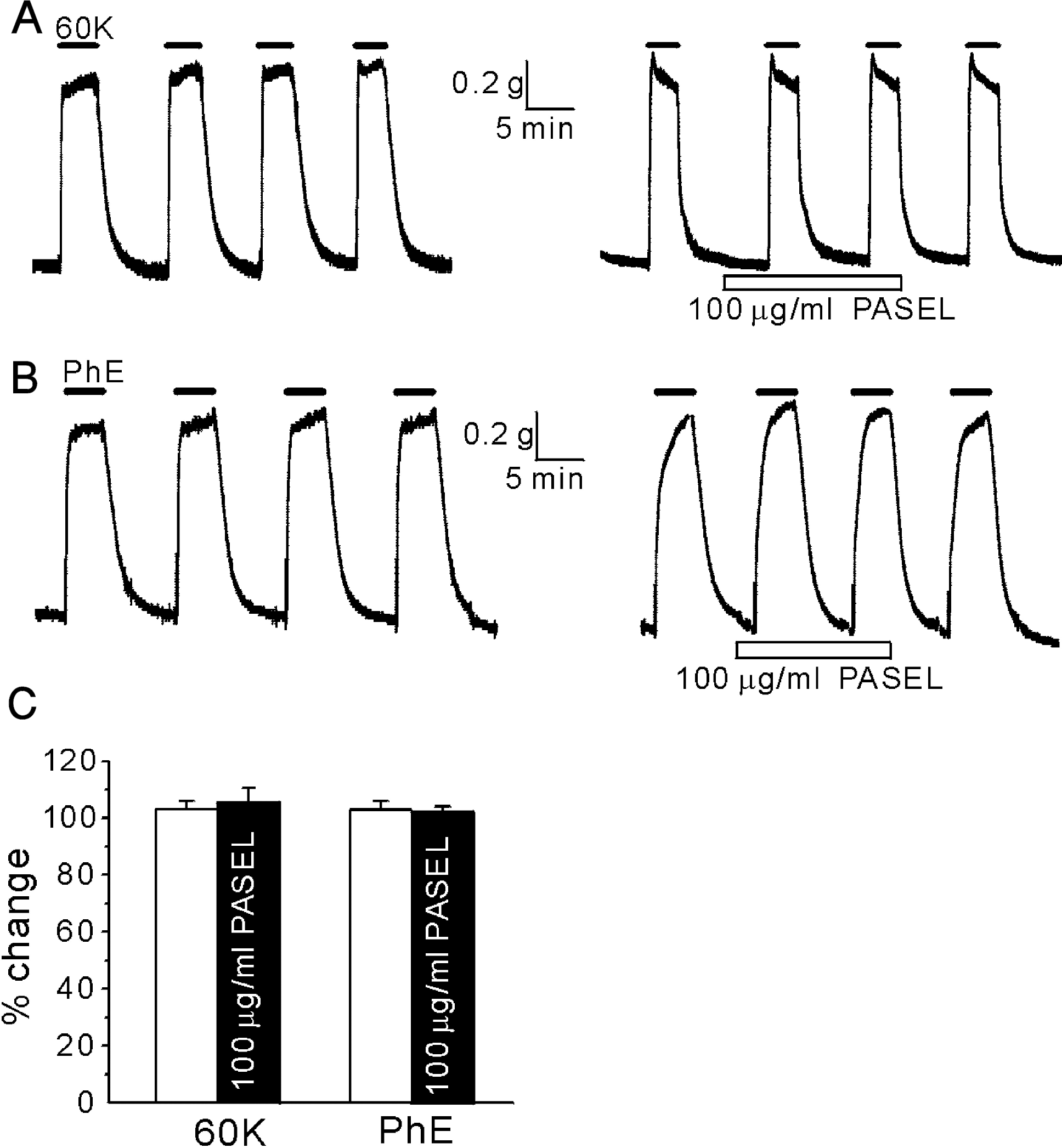
Fig. 3.
Effects of PASEL on MR of rat cerebral arteries. (A) A representative trace of diameter (Din) measured during application of PASEL (1~100 μg/ml) at 80 mmHg in the cerebral artery of WKR. (B) Summary of MR in % scale (see Methods) between WKR and SHR. There was no statistical significance between both groups. (C, D) Summary of the effects of 1~100 μg/ml PASEL on MR of WKR and SHR at 80 mmHg, respectively. Normalized diameters (% values of control Din at 80 mmHg) were averaged. The numbers in parenthesis above each point indicate numbers of tested arteries. (E, F) Summary of the effects of 10~100 μg/ml PASEL on MR of WKR and SHR at 120 mmHg, respectively (∗p value < 0.05).
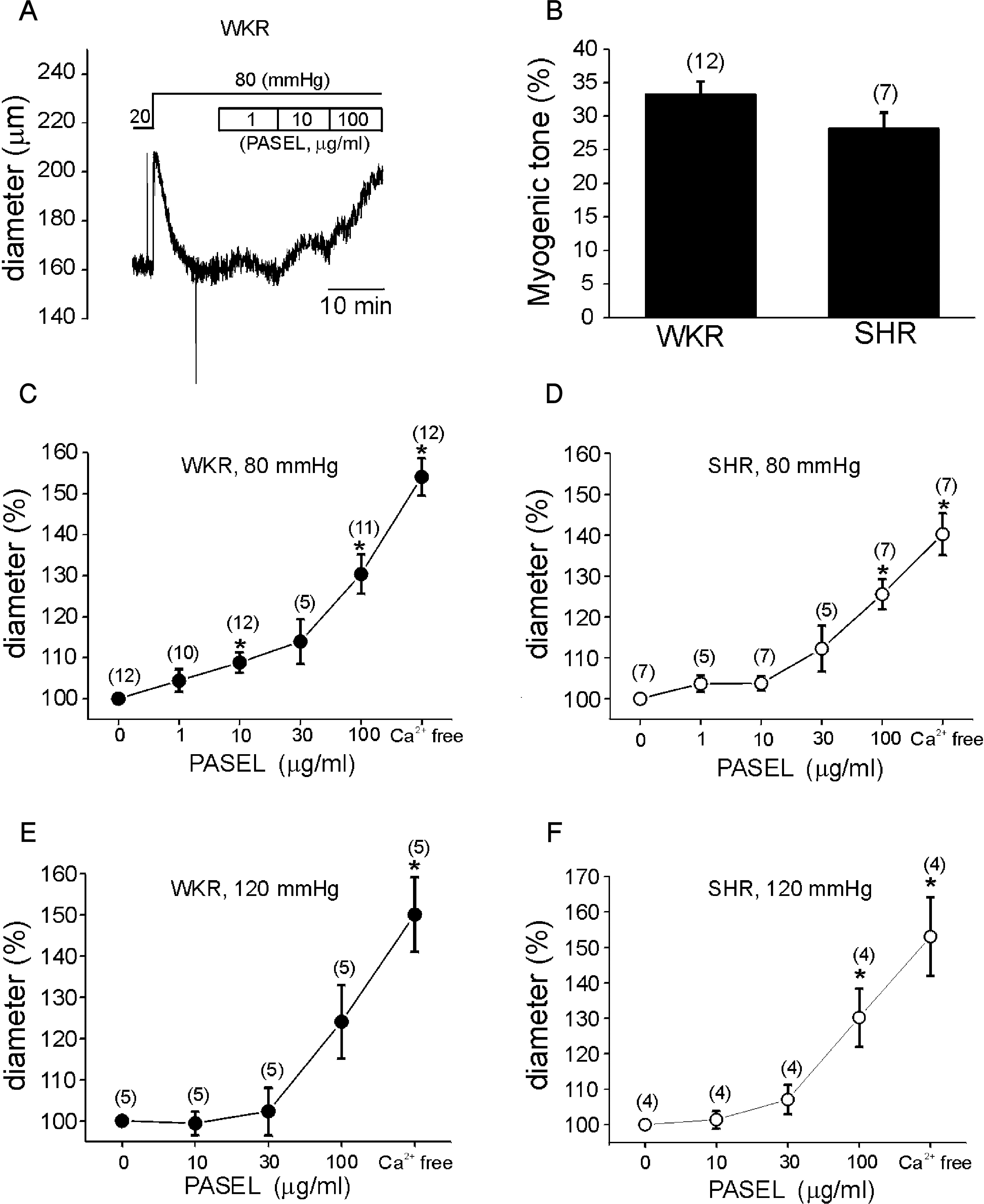
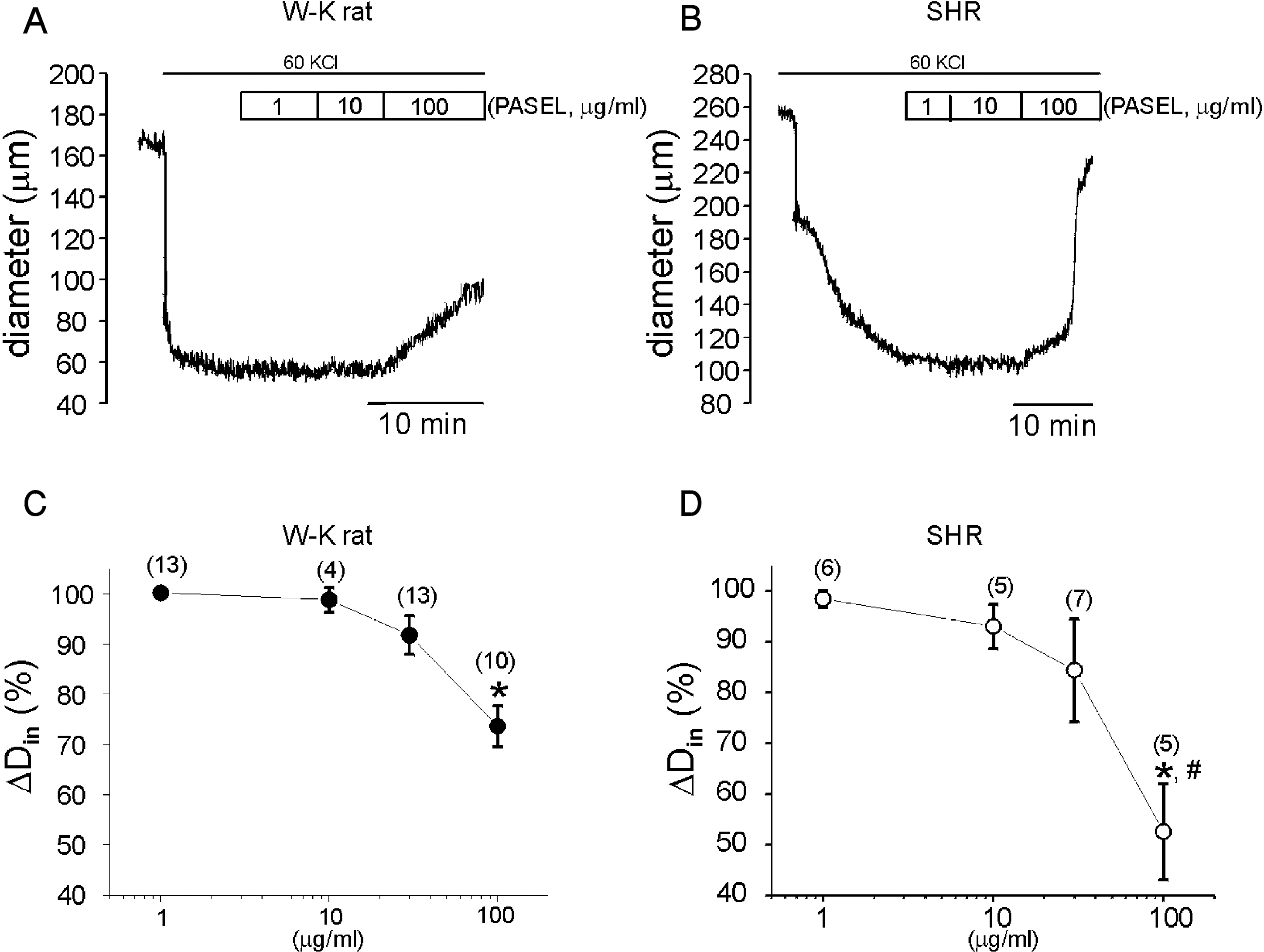
Fig. 4.
Effects of PASEL on high K+-induced constriction of rat cerebral arteries. (A, B) Representative traces of inner diameter (Din) showing the high K+-constriction and the inhibition by PASEL (1~100 μg/ml) treatment in WKR and SHR. (C, D) Summary of the concentration-dependent effects of PASEL in WKR and SHR, respectively. Changes in Din (ΔDin%) are expressed in percent values versus the extent of high-K+ constriction ΔDin at 40 mmHg as 100%. The extent of dilation by 100 μg/ml PASEL was more prominent in SHR than WKR (p<0.05, # in D).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download