Abstract
The effects of oxidized low-density lipoprotein (OxLDL) and its major lipid constituent lysophosphatidylcholine (LPC) on Ca2+ entry were investigated in cultured human umbilical endothelial cells (HUVECs) using fura-2 fluorescence and patch-clamp methods. OxLDL or LPC increased intracellular Ca2+ concentration ([Ca2+]i), and the increase of [Ca2+]i by OxLDL or by LPC was inhibited by La3+ or heparin. LPC failed to increase [Ca2+]i in the presence of an antioxidant tempol. In addition, store-operated Ca2+ entry (SOC), which was evoked by intracellular Ca2+ store depletion in Ca2+-free solution using the sarcoplasmic reticulum Ca2+ pump blocker, 2, 5-di-t-butyl-1, 4-benzohydroquinone (BHQ), was further enhanced by OxLDL or by LPC. Increased SOC by OxLDL or by LPC was inhibited by U73122. In voltage-clamped cells, OxLDL or LPC increased [Ca2+]i and simultaneously activated non-selective cation (NSC) currents. LPC-induced NSC currents were inhibited by 2-APB, La3+ or U73122, and NSC currents were not activated by LPC in the presence of tempol. Furthermore, in voltage-clamped HUVECs, OxLDL enhanced SOC and evoked outward currents simultaneously. Clamping intracellular Ca2+ to 1 μM activated large-conductance Ca2+-activated K+ (BKCa) current spontaneously, and this activated BKCa current was further enhanced by OxLDL or by LPC. From these results, we concluded that OxLDL or its main component LPC activates Ca2+-permeable Ca2+-activated NSC current and BKCa current simultaneously, thereby increasing SOC.
REFERENCES
Berliner JA., Heinecke JW. The role of oxidized lipoproteins in atherogenesis. Free Radic Biol Med. 20:707–727. 1996.

Fearon IM. OxLDL enhances L-type Ca2+ currents via lysophosphatidylcholine-induced mitochondrial reactive oxygen species (ROS) production. Cardiovasc Res. 69:855–864. 2006.
Hessler JR., Morel DW., Lewis LJ., Chisolm GM. Lipoprotein oxidation and lipoprotein-induced cytotoxicity. Arteriosclerosis. 3:215–222. 1983.

Itagaki K., Hauser CJ. Sphingosine 1-phosphate, a diffusible Ca2+ influx factor mediating store-operated Ca2+ entry. J Biol Chem. 278:27540–27547. 2003.
Jabr RI., Yamazaki J., Hume JR. Lysophosphatidylcholine triggers intracellular Ca2+ release and activation of non-selective cation channels in renal arterial smooth muscle cells. Pflugers Arch. 439:495–500. 2000.
Kamouchi M., Droogmans G., Nilius B. Membrane potential as a modulator of the free intracellular Ca2+ concentration in agonist-activated endothelial cells. Gen Physiol Biophys. 18:199–208. 1999.
Kamouchi M., Mamin A., Droogmans G., Nilius B. Nonselective cation channels in endothelial cells derived from human umbilical vein. J Membr Biol. 169:29–38. 1999.

Kim MY., Liang GH., Kim JA., Kim YJ., Oh S., Suh SH. Sphingosine-1-phosphate activates BKCa channels independently of G protein-coupled receptor in human endothelial cells. Am J Physiol Cell Physiol. 290:C1000–1008. 2006.
Kuhlmann CR., Schafer M., Li F., Sawamura T., Tillmanns H., Waldecker B., Wiecha J. Modulation of endothelial Ca2+ -activated K+ channels by oxidized LDL and its contribution to endothelial proliferation. Cardiovasc Res. 60:626–634. 2003.
Matsubara M., Hasegawa K. Benidipine, a dihydropyridine-Ca2+ channel blocker, prevents lysophosphatidylcholine-induced injury and reactive oxygen species production in human aortic endothelial cells. Atherosclerosis. 178:57–66. 2005.
Mehta JL. The role of LOX-1, a novel lectin-like receptor for oxidized low density lipoprotein, in atherosclerosis. Canadian J Cardiol. 20(Suppl B):32B–36B. 2004.
Mehta JL., Chen J., Hermonat PL., Romeo F., Novelli G. Lectin-like, oxidized low-density lipoprotein receptor-1 (LOX-1): a critical player in the development of atherosclerosis and related disorders. Cardiovasc Res. 69:36–45. 2006.

Muller J., Petkovic M., Schiller J., Arnold K., Reichl S., Arnhold J. Effects of lysophospholipids on the generation of reactive oxygen species by fMLP- and PMA-stimulated human neutrophils. Luminescence. 17:141–149. 2002.

Nilius B. Permeation properties of a non-selective cation channel in human vascular endothelial cells. Pflugers Arch. 416:609–611. 1990.

Nilius B., Oike M., Zahradnik I., Droogmans G. Activation of a Cl− current by hypotonic volume increase in human endothelial cells. J Gen Physiol. 103:787–805. 1994.
Nilius B., Schwarz G., Oike M., Droogmans G. Histamine- activated, non-selective cation currents and Ca2+ transients in endothelial cells from human umbilical vein. Pfluers Archiv. 424:285–293. 1993.
Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 362:801–809. 1993.

Steinberg D. Low density lipoprotein oxidation and its pathobiological significance. J Biol Chem. 272:20963–20966. 1997.

Steinberg D., Parthasarathy S., Carew TE., Khoo JC., Witztum JL. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 320:915–924. 1989.
Steinbrecher UP., Zhang HF., Lougheed M. Role of oxidatively modified LDL in atherosclerosis. Free Radic Biol Med. 9:155–168. 1990.

Suh SH., Watanabe H., Droogmans G., Nilius B. ATP and nitric oxide modulate a Ca2+-activated non-selective cation current in macrovascular endothelial cells. Pflugers Arch. 444:438–445. 2002.
Takeshita S., Inoue N., Gao D., Rikitake Y., Kawashima S., Tawa R., Sakurai H., Yokoyama M. Lysophosphatidylcholine enhances superoxide anions production via endothelial NADH/NADPH oxidase. J Atheroscler Thromb. 7:238–246. 2000.

Terasawa K., Nakajima T., Iida H., Iwasawa K., Oonuma H., Jo T., Morita T., Nakamura F., Fujimori Y., Toyo-oka T., Nagai R. Nonselective cation currents regulate membrane potential of rabbit coronary arterial cell: modulation by lysophosphatidy-lcholine. Circulation. 106:3111–3119. 2002.
Thorin E., Hamilton C., Dominiczak AF., Dominiczak MH., Reid JL. Oxidized-LDL induced changes in membrane physico- chemical properties and [Ca2+]i of bovine aortic endothelial cells. Influence of vitamin E. Atherosclerosis. 114:185–195. 1995.
van Tits LJ., Hak-Lemmers HL., Demacker PN., Stalenhoef AF., Willems PH. Oxidized low-density lipoprotein induces Ca2+ influx in polymorphonuclear leukocytes. Free Radic Biol Med. 29:747–755. 2000.
Voets T., Wei L., De Smet P., van Driessche W., Eggermont J., Droogmans G., Nilius B. Downregulation of volume-activated Cl− currents during muscle differentiation. Am J Physiol. 272:C667–674. 1997.
Weisser B., Locher R., Mengden T., Vetter W. Oxidation of low density lipoprotein enhances its potential to increase intracellular free calcium concentration in vascular smooth muscle cells. Arterioscler Thromb. 12:231–236. 1992.

Young IS., McEneny J. Lipoprotein oxidation and atherosclerosis. Biochem Soc Trans. 29:358–362. 2001.

Fig. 1.
Effect of OxLDL or LPC on intracellular Ca2+ concentration ([Ca2+]i) in HUVECs. (A, C and D) OxLDL-induced increase of [Ca2+]i in the presence of (A, C) or in the absence of extracellular Ca2+ (D). (B) in the presence of an antioxidant tempol, LPC failed to increase [Ca2+]i. (E and F) The increase of store-operated Ca2+ entry by OxLDL (E) or LPC (F).
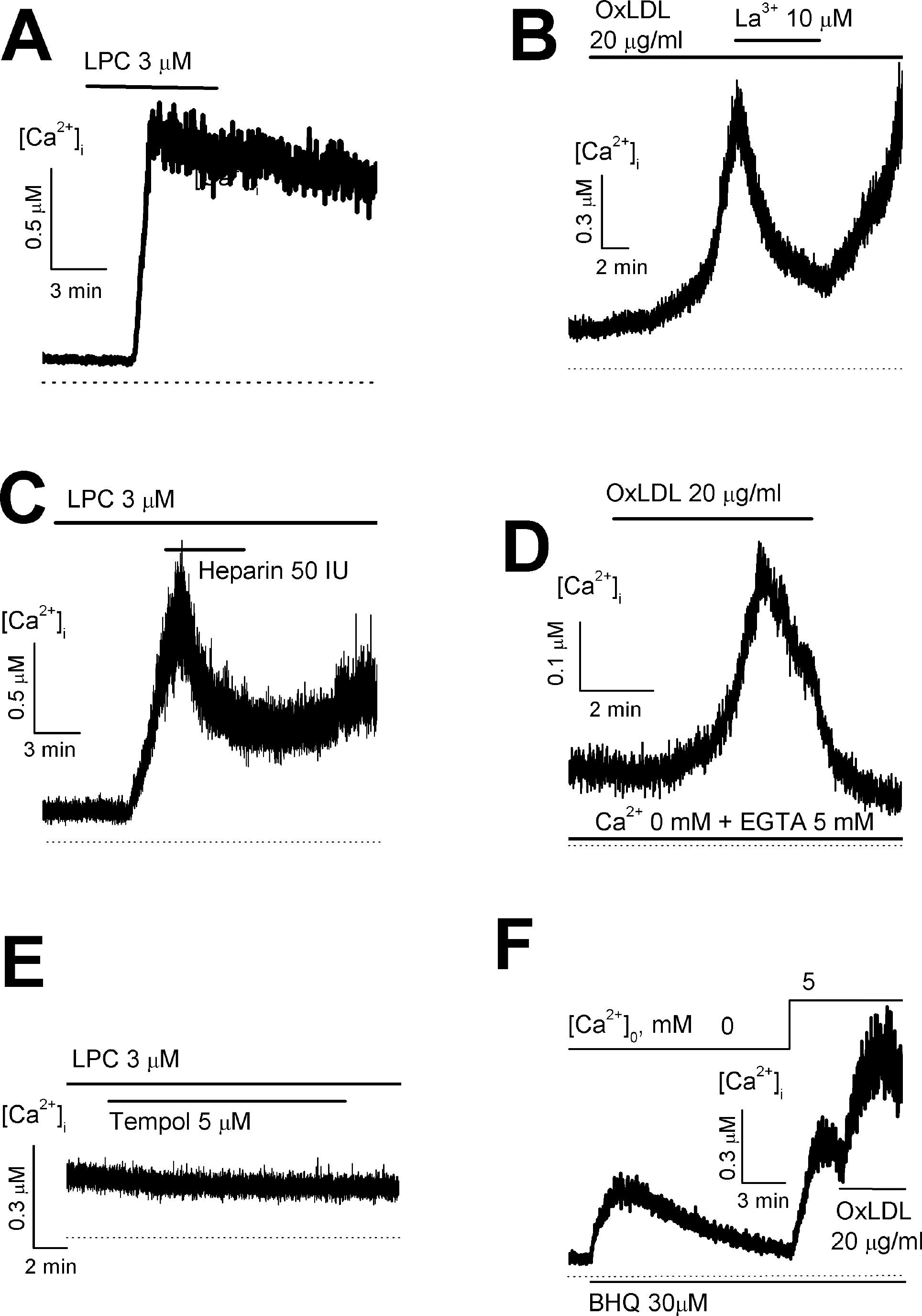
Fig. 2.
Effect of OxLDL on store-operated Ca2+ entry in a voltage-clamped cell. Membrane currents and [Ca2+]i were measured simultaneously at a holding potential of 0 mV in nystatin-perforated mode. (A) further activation of store-operated Ca2+ entry (SOC) by OxLDL. SOC was activated by depletion of the intracellular Ca2+ store and re-exposure to Ca2+. (B) time course of membrane currents at +50 and −50 mV activated by a rise in [Ca2+]i (A). Membrane currents were activated by repetitive ramps from −100 to +100 mV. (C) current-voltage relations obtained at points indicated 1~3 in (B).
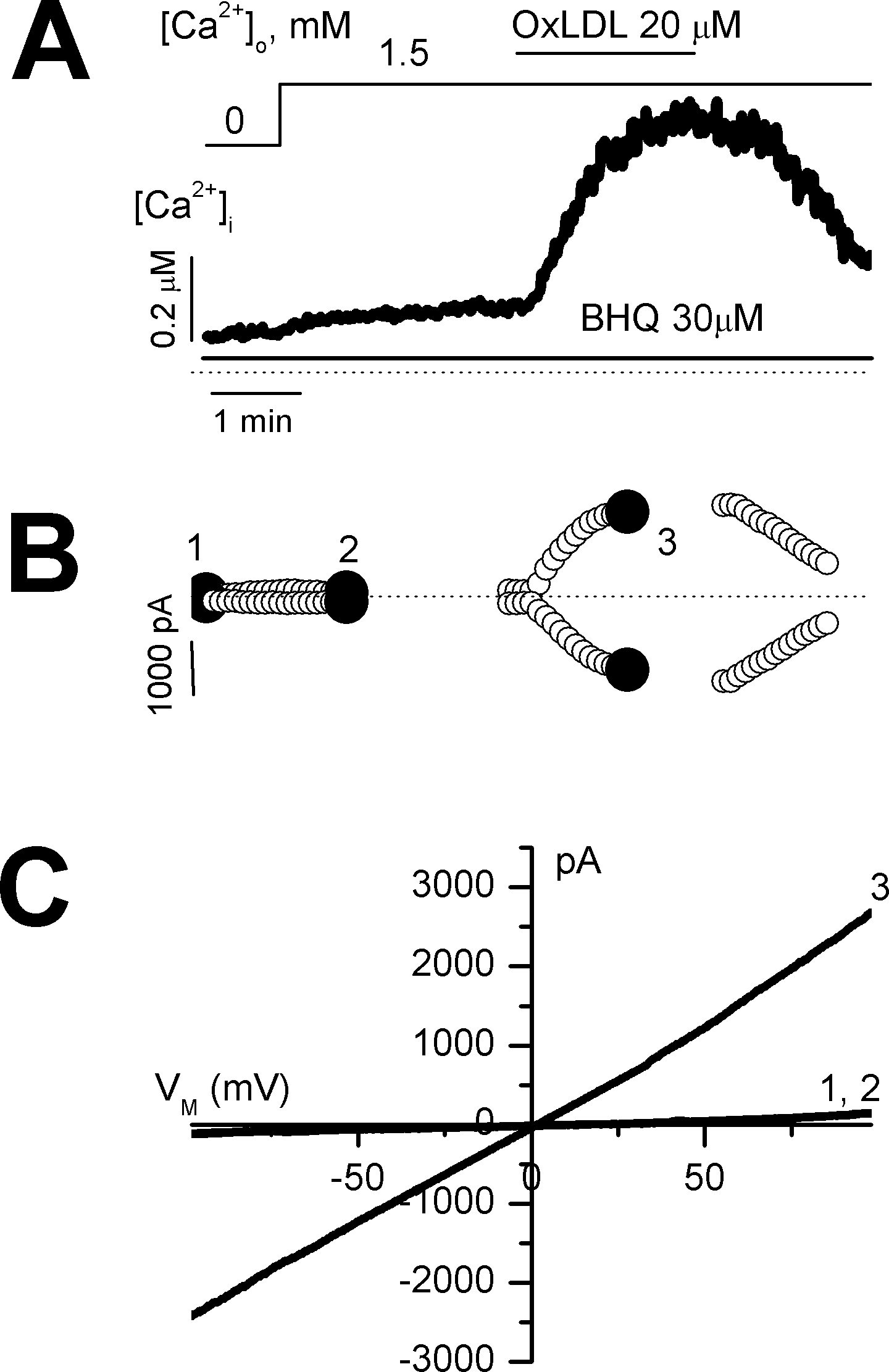
Fig. 3.
Effect of LPC on nonselective cation (NSC) currents in HUVECs. Membrane potential was held at 0 mV. (A, C and E) time courses of membrane currents obtained at +50 and −50 mV during repetitive ramps from −100 to +100 mV. (B, D and F) current-voltage relations obtained at points indicated 1~9 in (A), (C) and (E), respectively. (G) bar graph showing current density at +50 mV. n=8, ∗p < 0.001.
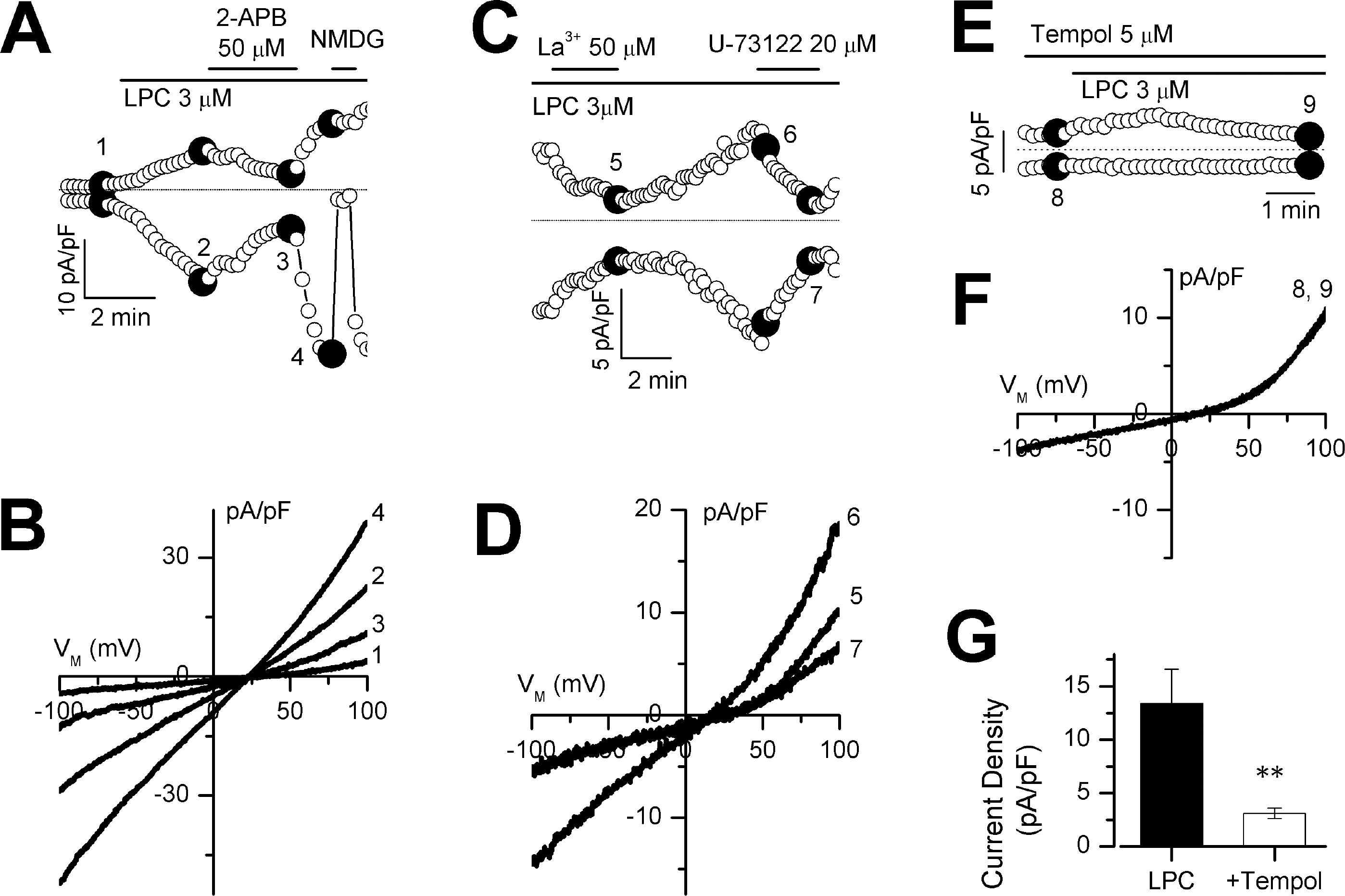
Fig. 4.
Effect of OxLDL on store-operated Ca2+ entry and membrane currents in a voltage-clamped cell. Membrane currents (B) and [Ca2+]i (A) were measured simultaneously at a holding potential of 0 mV in nystatin-perforated mode.
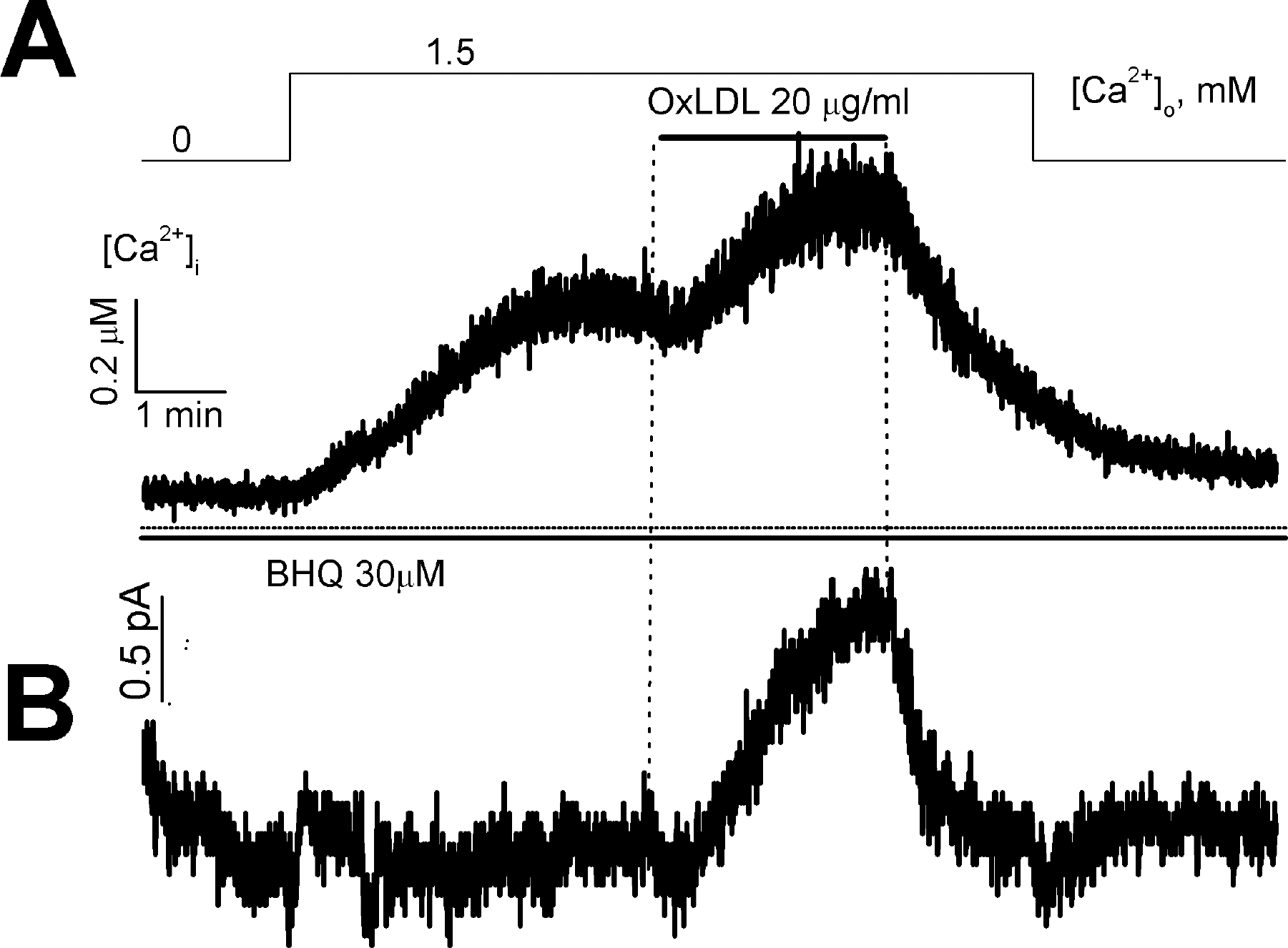
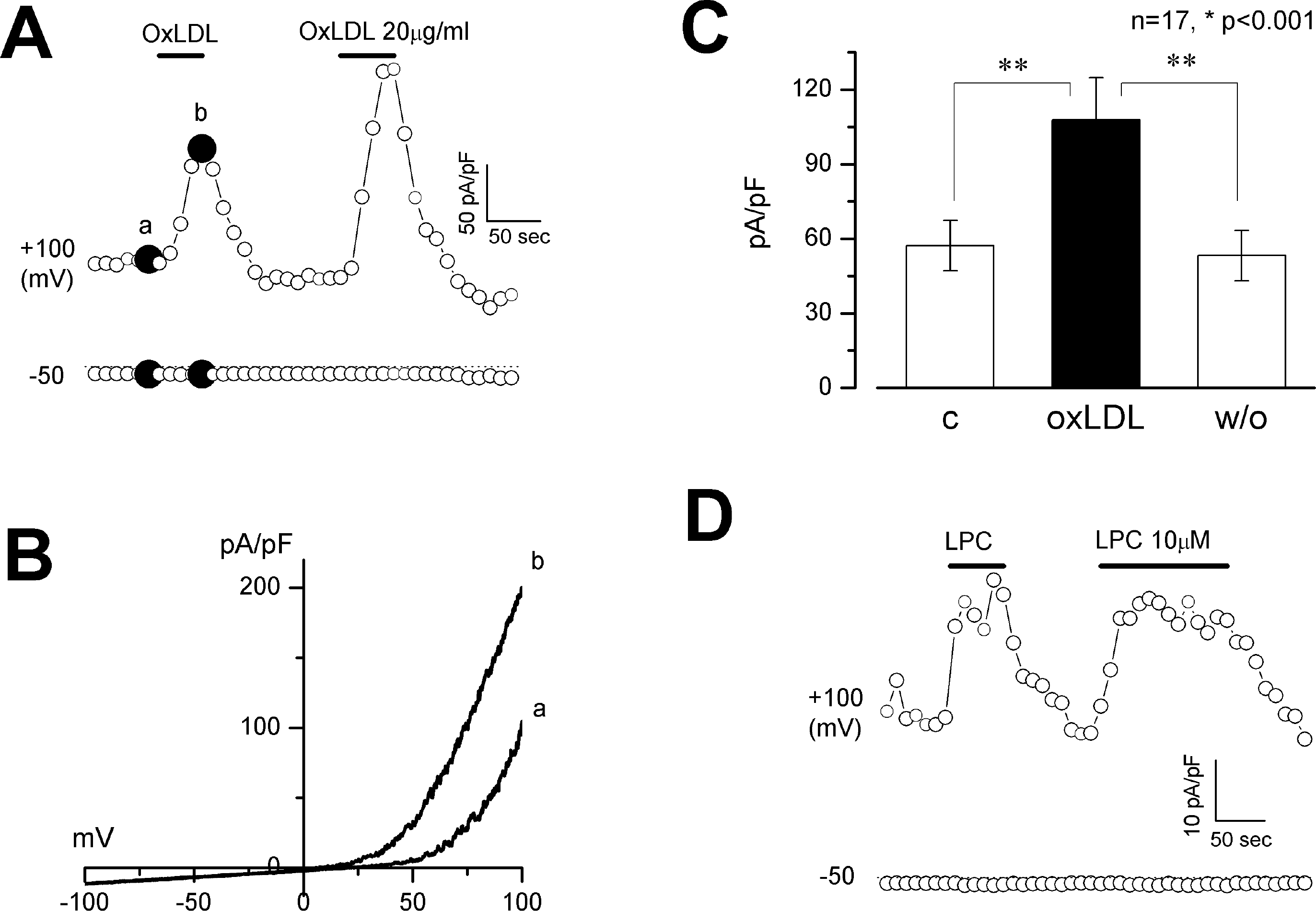
Fig. 5.
Effect of OxLDL and LPC on BKCa currents. BKCa currents were activated by clamping [Ca2+]i at 1 (A-C) or 0.5 μM (D). Membrane potential was held at 0 mV. (A and D) time course of membrane current change by OxLDL (A) or LPC (D) measured at −50 and +100 mV. (B) current-voltage relationship obtained from the voltage ramps from −100 to +100 mV labeled as a and b in (A). (C) bar graph showing current density at +100 mV. (C) control. OxLDL, maximal current density obtained during OxLDL (20 μg/ml) application; w/o, wash-out. n=17, ∗p <0.001.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download