Abstract
Previous observations suggest that Bis, a Bcl-2-binding protein, may play a role the neuronal and glial differentiation in vivo. To examine this further, we investigated Bis expression during the in vitro differentiation of P19 embryonic carcinoma cells induced by retinoic acid (RA). Western blotting and RT-PCR assays showed that Bis expression was temporarily decreased during the free floating stage and then began to increase on day 6 after the induction of differentiation. Double immunostaining indicated that Bis-expressing cells do not express several markers of differentiation, including NeuN, MAP-2 and Tuj-1. However, some of the Bis-expressing cells also were stained with GFAP-antibodies, indicating that Bis is involved glial differentiation. Using an shRNA strategy, we developed bis-knock down P19 cells and compared them with control P19 cells for the expression of NeuroD, Mash-1 and GFAP during RA-induced differentiation. Among these, only GFAP induction was significantly attenuated in P19-dnbis cells and the population showing GFAP immunoreactivity was also decreased. It is noteworthy that distribution of mature neurons and migrating neurons was disorganized, and the close association of migrating neuroblasts with astrocytes was not observed in P19-dnbis cells. These results suggest that Bis is involved in the migration-inducing activity of glial cells.
REFERENCES
Bonelli P., Petrella A., Rosati A., Romano MF., Lerose R., Pagliuca MG., Amelio T., Festa M., Martire G., Venuta S., Turco MC., Leone A. BAG3 protein regulates stress-induced apoptosis in normal and neoplastic leukocytes. Leukemia. 18:358–360. 2004.

Carra S., Seguin SJ., Lambert H., Landry J. HspB8 chaperone activity toward poly(Q)-containing proteins depends on its association with Bag3, a stimulator of macroautophagy. J Biol Chem. 283:1437–1444. 2008.

Chen L., Wu W., Dentchev T., Zeng Y., Wang J., Tsui I., Tobias JW., Bennett J., Baldwin D., Dunaief JL. Light damage induced changes in mouse retinal gene expression. Exp Eye Res. 79:239–247. 2004.

Choi JS., Lee JH., Kim HY., Chun MH., Chung JW., Lee MY. Developmental expression of Bis protein in the cerebral cortex and hippocampus of rats. Brain Res. 1092:69–78. 2006.

Choi JS., Lee JH., Shin YJ., Lee JY., Yun H., Chun MH., Lee MY. Transient expression of Bis protein in the midline glial structure in the developing rat brainstem and spinal cord. Cell Tissue Res. 337:27–36. 2009.
Doong H., Price J., Kim YS., Gasbarre C., Probst J., Liotta LA., Blanchette J., Rizzo K., Kohn E. CAIR-1/BAG-3 forms an EGF-regulated ternary complex with phospholipase C-gamma and Hsp70/Hsc70. Oncogene. 19:4385–4395. 2000.
Hong S., Heo J., Lee S., Heo S., Kim SS., Lee YD., Kwon M. Methyltransferase-inhibition interferes with neuronal differentiation of P19 embryonal carcinoma cells. Biochem Biophys Res Commun. 377:935–940. 2008.

Iwasaki M., Homma S., Hishiya A., Dolezal SJ., Reed JC., Takayama S. BAG3 regulates motility and adhesion of epithelial cancer cells. Cancer Res. 67:10252–10259. 2007.

Jacobs AT., Marnett LJ. HSF1-mediated BAG3 expression attenuates apoptosis in 4-hydroxynonenal-treated colon cancer cells via stabilization of anti-apoptotic Bcl-2 proteins. J Biol Chem. 284:9176–9183. 2009.

Jing XT., Wu HT., Wu Y., Ma X., Liu SH., Wu YR., Ding XF., Peng XZ., Qiang BQ., Yuan JG., Fan WH., Fan M. DIXDC1 promotes retinoic acid-induced neuronal differentiation and inhibits gliogenesis in P19 cells. Cell Mol Neurobiol. 29:55–67. 2009.

Jones-Villeneuve EM., McBurney MW., Rogers KA., Kalnins VI. Retinoic acid induces embryonal carcinoma cells to differentiate into neurons and glial cells. J Cell Biol. 94:253–262. 1982.

Jones-Villeneuve EM., Rudnicki MA., Harris JF., McBurney MW. Retinoic acid-induced neural differentiation of embryonal carcinoma cells. Mol Cell Biol. 3:2271–2279. 1983.

Kassis JN., Guancial EA., Doong H., Virador V., Kohn EC. CAIR-1/BAG-3 modulates cell adhesion and migration by downregulating activity of focal adhesion proteins. Exp Cell Res. 312:2962–2971. 2006.

Lee JH., Takahashi T., Yasuhara N., Inazawa J., Kamada S., Tsujimoto Y. Bis, a Bcl-2-binding protein that synergizes with Bcl-2 in preventing cell death. Oncogene. 18:6183–6190. 1999.

Lee MY., Kim SY., Shin SL., Choi YS., Lee JH., Tsujimoto Y. Reactive astrocytes express bis, a bcl-2-binding protein, after transient forebrain ischemia. Exp Neurol. 175:338–346. 2002.

Lim DA., Alvarez-Buylla A. Interaction between astrocytes and adult subventricular zone precursors stimulates neurogenesis. Proc Natl Acad Sci U S A. 96:7526–7531. 1999.

Liour SS., Yu RK. Differentiation of radial glia-like cells from embryonic stem cells. Glia. 42:109–117. 2003.

Pagliuca MG., Lerose R., Cigliano S., Leone A. Regulation by heavy metals and temperature of the human BAG-3 gene, a modulator of Hsp70 activity. FEBS Lett. 541:11–15. 2003.

Park HJ., Choi JS., Chun MH., Chung JW., Jeon MH., Lee JH., Lee MY. Immunohistochemical localization of Bis protein in the rat central nervous system. Cell Tissue Res. 314:207–214. 2003.

Romano MF., Festa M., Pagliuca G., Lerose R., Bisogni R., Chiurazzi F., Storti G., Volpe S., Venuta S., Turco MC., Leone A. BAG3 protein controls B-chronic lymphocytic leukaemia cell apoptosis. Cell Death Differ. 10:383–385. 2003.

Fig. 1.
Expression of Bis in GFAP-positive glia cells during RA-induced differentiation in P19 cells. (A) Light microscopic images of P19 cells before (left) and after (right) the induction of neuronal differentiation (day 12). (B) Change in Bis protein levels during the RA-induced differentiation of P19 cells was examined by immunoblotting (upper panel). The expression of GAPDH is shown as a loading control. The relative expression of bis mRNA levels was determined by real-time RT-PCR after normalizing to endogenous GAPDH control (lower graph). The value at day 0 was arbitrarily designated as 1.0. (C) Confocal laser microscopic imaging of immunofluorescence for Bis (e-h) and one of the marker antibodies for NeuN (a), MAP-2 (b), Tuj-1 (c) and GFAP (d) at day 12 after the induction of neuronal differentiation. (i-l) Superimposed images of FITC (a-d) and Cy3 (e-h). Scale bar, 50 μm. (D) Higher magnification of the boxed area in (Cl). Most GFAP labeled cells were immunoreactive for Bis (arrows in panel a-c). Scale bar, 20 μm.
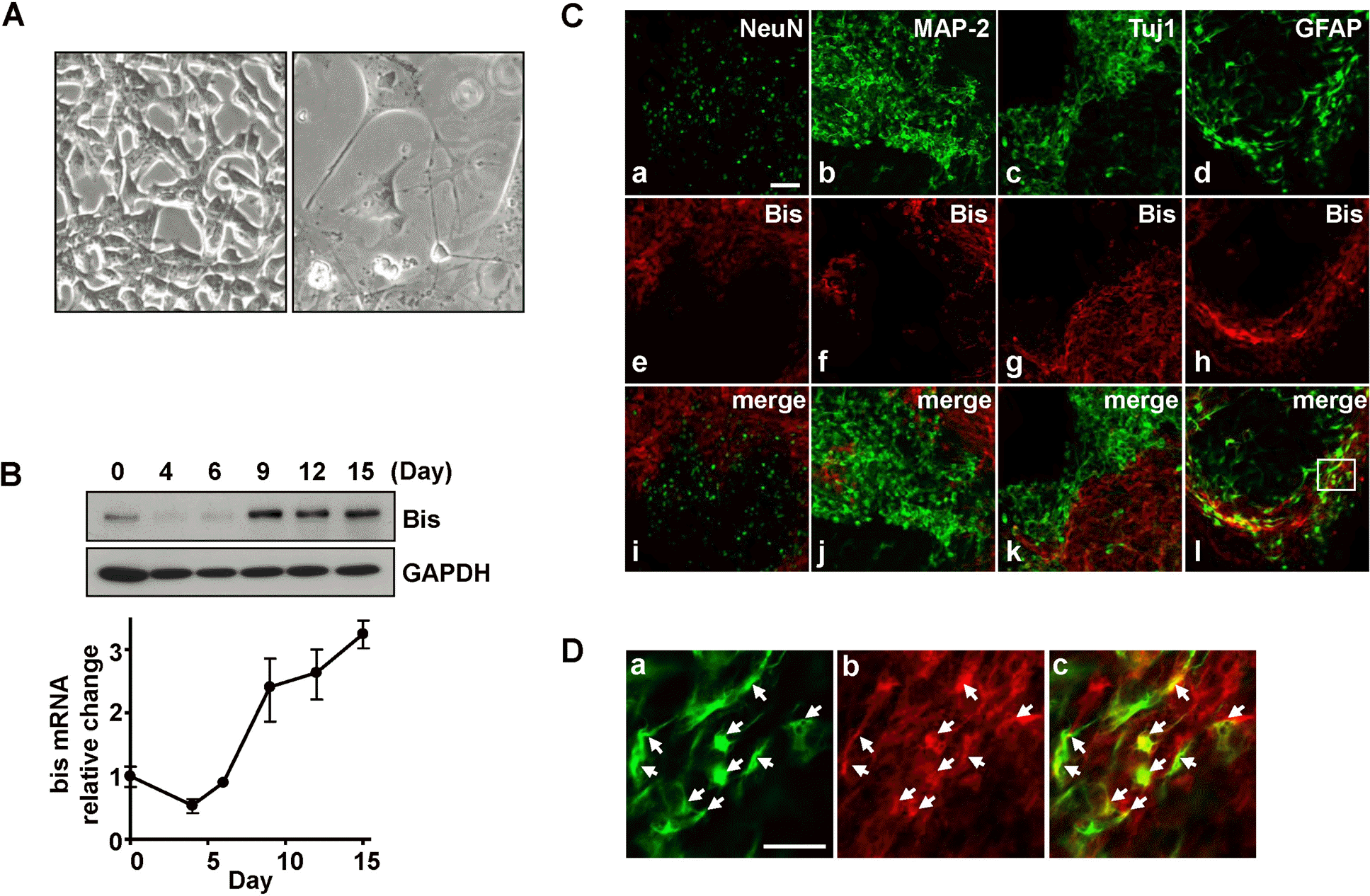
Fig. 2.
Bis-knock down decreased the induction of GFAP in P19 cells after RA-induced differentiation. (A) Change in mRNA levels of Bis, NeuroD, Mash-1 and GFAP during RA-induced differentiation was examined by RT- PCR in P19-con and P19-dnbis cells. Note that GFAP induction was significantly decreased in P19-dnbis cells. (B) The expression of protein levels of Bis and GFAP was determined by immunoblotting in P19-con and P19-dnbis cells after the induction of differentiation with RA.
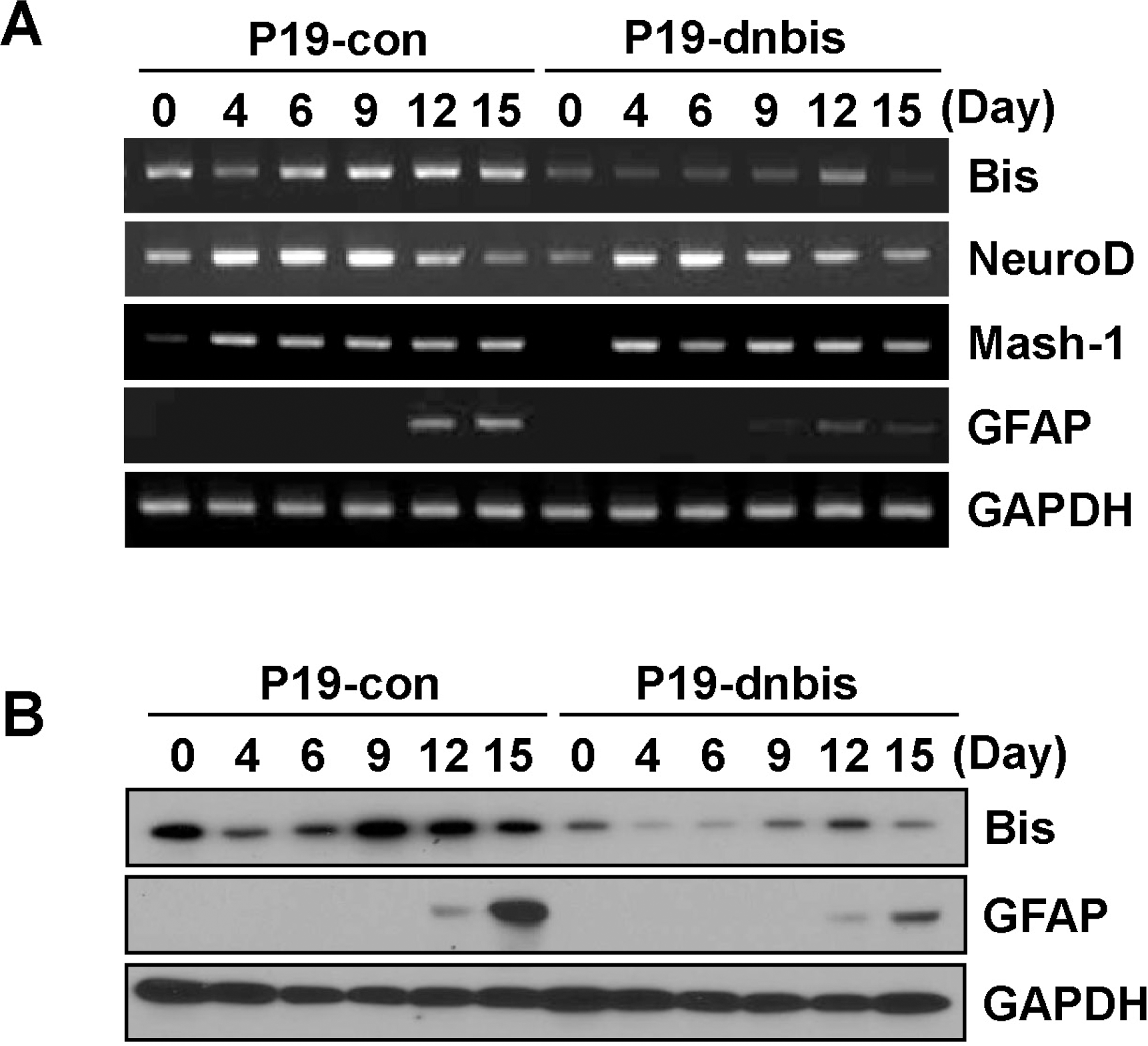
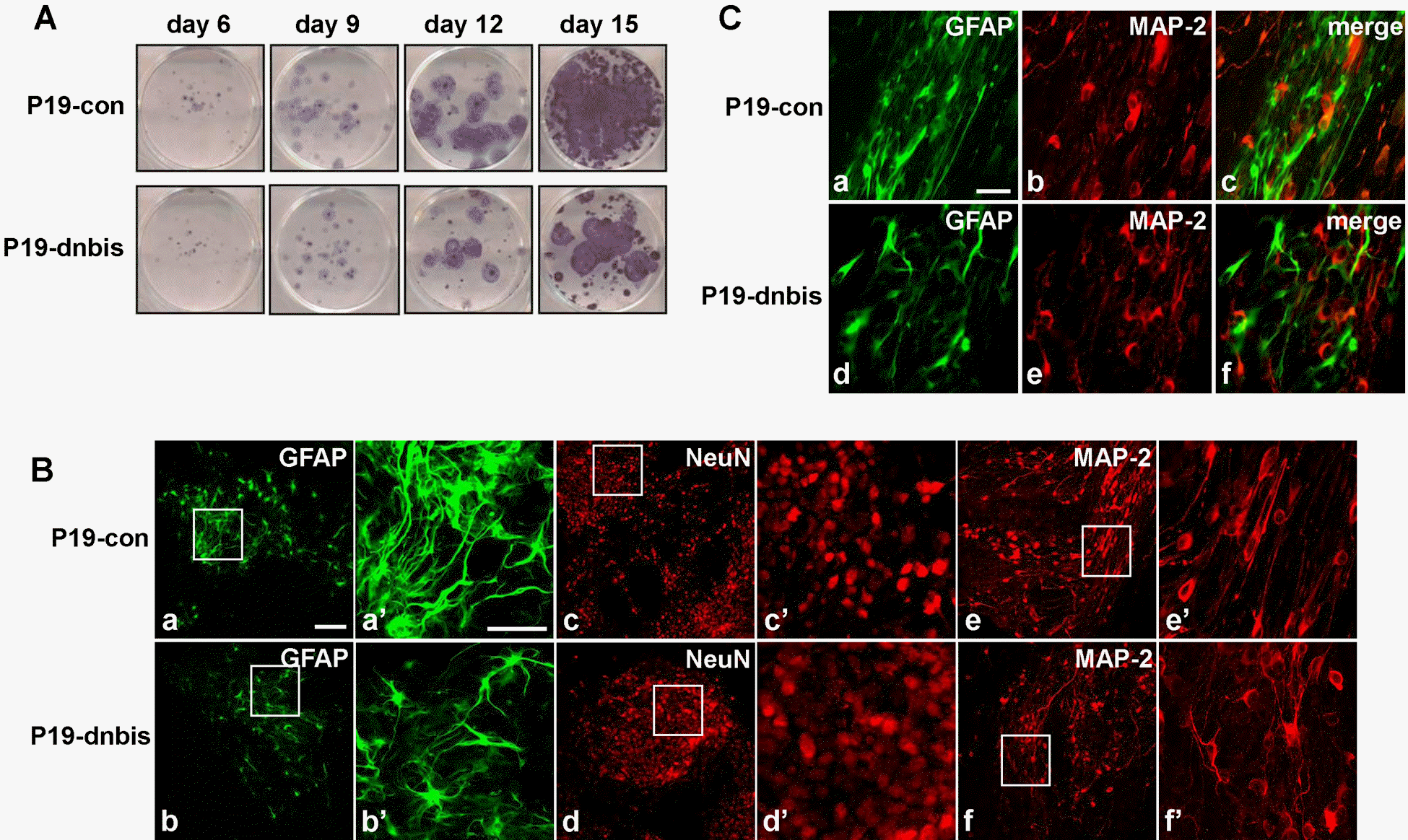
Fig. 3.
The impairment of glial differentiation and neuronal distribution in Bis-knock down P19 cells after RA-induced differentiation. (A) The staining of viable cells after the induction of differentiation with RA in P19-con and P19-dnbis cells. After culturing cell aggregates in a Petri dish for 4 days, the cells were re-plated in 6 well tissue culture dishes and stained with MTT solution at the indicated days. (B) Confocal laser microscopic imaging of immunofluorescence for GFAP (a, b), NeuN (c, d) and MAP-2 (e, f) of P19-con (upper panels) and P19-dnbis (lower panels). a', b', c', d', e’ and f’ are higher magnifications of boxed areas of a, b, c, d, e and f, respectively. Scale bar, 50 μm (a-f) and 20 μm (a'-f'). (C) Confocal laser microscopic imaging of immunofluorescence for GFAP (a, d), MAP-2 (b, e) and overlay (c, f) of P19-con (upper panels) and P19-dnbis (lower panels) at day 12 after the induction of neuronal differentiation. Scale bar, 20 μm.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download