Abstract
This study was designed to clarify the mechanism of the inhibitory effect of forskolin on contraction, cytosolic Ca2+ level ([Ca2+]i), and Ca2+ sensitivity in guinea pig ileum. Forskolin (0.1 nM~10 μM) inhibited high K+ (25 mM and 40 mM)- or histamine (3 μM)-evoked contractions in a concentration-dependent manner. Histamine-evoked contractions were more sensitive to forskolin than high K+-evoked contractions. Spontaneous changes in [Ca2+]i and contractions were inhibited by forskolin (1 μM) without changing the resting [Ca2+]i. Forskoln (10 μ M) inhibited muscle tension more strongly than [Ca2+]i stimulated by high K+, and thus shifted the [Ca2+]i-tension relationship to the lower-right. In histamine-stimulated contractions, forskolin (1 μ M) inhibited both [Ca2+]i and muscle tension without changing the [Ca2+]i-tension relationship. In α-toxin-permeabilized tissues, forskolin (10 μM) inhibited the 0.3 μ M Ca2+-evoked contractions in the presence of 0.1 mM GTP, but showed no effect on the Ca2+-tension relationship. We conclude that forskolin inhibits smooth muscle contractions by the following two mechanisms: a decrease in Ca2+ sensitivity of contractile elements in high K+-stimulated muscle and a decrease in [Ca2+]i in histamine-stimulated muscle.
REFERENCES
Adelstein RS., Conti MA., Hathaway DR., Klee CB. Phosphorylation of smooth muscle myosin light chain kinase by the catalytic subunit of adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 253:8347–8350. 1978.
Ahn HY., Kang SE., Chang KC., Karaki H. Dibutyryl cyclic AMP and forskolin inhibit phosphatidyl-inositol hydrolysis, Ca2+ influx and contraction in vascular smooth muscle. Jpn J Pharmacol. 59:263–265. 1992.
Andersson R., Nilsson K. Role of cyclic nucleotides: metabolism and mechanical activity in smooth muscle. In Biochemistry of Smooth Muscle (edited by Stephens NL), 263−291. 1977.
Bhalla RC., Webb RC., Singh D., Brock T. Role of cyclic AMP in rat aortic microsomal phosphorylation and calcium uptake. Am J Physiol. 234:H508–H514. 1978.

Bulbring E., Tomita T. Catecholamine action on smooth muscle. Pharmacol Rev. 39:49–96. 1987.
Cooper DM. Molecular and cellular requirements for the regulation adenylate cyclases by calcium. Biochem Soc Trans. 31:912–915. 2003.
Eckly-Michel A., Martin V., Lugnier C. Involvement of cyclic nucleotide-dependent protein kinases in cyclic AMP-mediated vasorelaxation. Br J Pharmacol. 122:158–164. 1997.

Hall IP., Donaldson J., Hill SJ. Inhibition od histamine-stimulated inositol phospholipids hydrolysis by agents which increase cyclic AMP levels in bovine tracheal smooth muscle. Br J Pharmacol. 97:603–613. 1989.
Ise S., Nishimura J., Hirano K., Hara N., Kanaide H. Theophylline attenuates Ca2+ sensitivity and modulates BK channels in porcine tracheal smooth muscle. Br J Pharmacol. 140:939–947. 2003.
Karaki H. Localization and sensitivity in vascular smooth muscle. Trends Pharmacol Sci. 10:320–325. 1989.
Kwon SC., Ozaki H., Hori M., Karaki H. Isoproterenol changes the relationship between cytosolic Ca2+ and contraction in guineapig taneia caecum. Jpn J Pharmacol. 61:57–64. 1993.
Kwon SC., Ozaki H., Karaki H. NO donor sodium nitroprusside inhibits excitation-contraction coupling in guinea pig taenia coli. Am J Physiol. 279:G1235–G1241. 2000a.

Kwon SC., Park KY., Ahn DS., Lee HY., Kang BS. The effect of NO donor on contraction, cytosolic Ca2+ level and ionic currents in guinea-pig ileal smooth muscle. Korean J Physiol Pharmacol. 4:33–40. 2000b.
Kushida M., Takeuchi T., Fujita A., Hata F. Dependence of Ca2+- induced contraction in a-toxin-permeabilized preparations of rat femoral artery. J Pharmacol Sci. 93:171–179. 2003.
Lincoln TM., Cornwell TL. Towards an understanding of the mechanism of action of cyclic AMP and cyclic GMP in smooth muscle relaxation. Blood Vessels. 28:129–137. 1991.

Morales S., Camello PJ., Mawe GM., Pozo MJ. Cyclic AMP-mediated inhibition of gallbladder contractility: role of K+ channel activation and Ca2+ handling. Br J Pharmacol. 143:994–1005. 2004.
Murray KJ. Cyclic AMP and mechanism of vasodilation. Pharmacol Ther. 47:329–354. 1990.
Murthy KS. Signaling for contraction and relaxation in smooth muscle of the gut. Annu Rev Physiol. 68:345–374. 2006.

Nishikori K., Maeno H. Close relationship between adenosine3': 5'-monophosphate-dependent endogenous phosphorylation of a specific protein and stimulation of calcium uptake in rat uterine microsomes. J Biol Chem. 254:6099–6106. 1979.
Nishimura J., van Breemen C. Direct regulation of smooth muscle contractile elements by second messengers. Biochem Biophys Res Commun. 163:929–935. 1989.

Ozaki H., Blondfield DP., Hori M., Sanders KM., Publicover NG. Cyclic AMP-mediated regulation of excitation-contraction coupling in canine gastric smooth muscle. J Physiol. 447:351–372. 1992.

Ozaki H., Hori M., Kim YS., Kwon SC., Ahn DS., Nakazawa H., Kobaysahi M., Karaki H. Inhibitory mechanism of xestospongin-C on contraction and ion channels in the intestinal smooth muscle. Br J Pharmacol. 137:1207–1212. 2002.

Pfitzer G., Hofmann F., DiSalvo J., Ruegg JC. cGMP and cAMP inhibit tension development in skinned coronary arteries. Pflugers Arch. 401:277–280. 1984.

Porter M., Evans MC., Miner AS., Berg KM., Ward KR., Ratz PH. Convergence of Ca2+-desensitizing mechanisms activated by forskolin and phenylephrine, but not 8-bromo-cGMP. Am J Physiol. 290:C1552–C1559. 2006.
Ratz PH. Receptor activation induces short-term modulation of arterial sontractions: memory in vascular smooth muscle. Am J Physiol. 269:C417–C423. 1999.
Rembold CM., Chen XL. Mechanisms responsible for forskolin-induced relaxation of rat tail artery. Hypertension. 31:872–877. 1998.

Rembold CM., O'Connor MJ., Clarkson M., Wardle RL., Murphy RA. HSP20 phosphorylation in nitroglycerin- and forskolin-induced sustained reductions in swine carotid artery. J Appl Physiol. 91:1460–1466. 2001.
Seamon KB., Daly JW. Forskolin: its biological and chemical properties. Adv Cyclic Nucleotide Protein Phosphoryl Res. 20:1–150. 1986.
Wellman GC., Santana LF., Bonev AD., Nelson MT. Role of phospholamban in the modulation of arterial Ca2+ sparks and Ca2+-activated K+ channels by cAMP. Am J Physiol. 281:C1029–C1037. 2001.
Fig. 1.
Concentration-response relationship for the inhibitory effect of forskolin on contractions induced by 25 mM KCl, 40 mM KCl, and 3 μM histamine. Forskolin (0.1 nM~10 μM) was cumulatively added after the contractions reached a steady state.
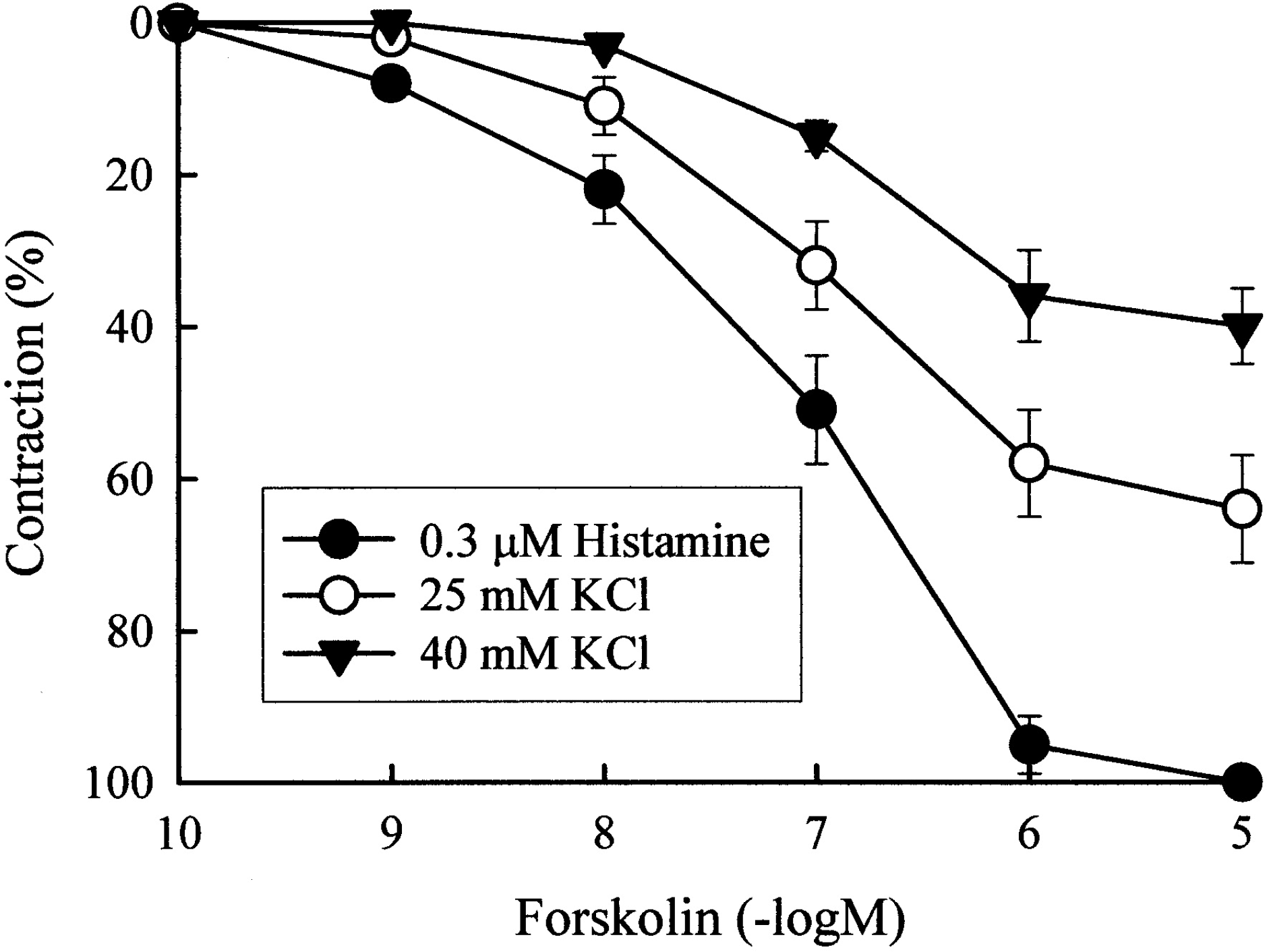
Fig. 2.
The effect of forskolin (1 μM) on [Ca2+]i (upper trace) and muscle tension (lower trace) in spontaneously active ileum. 100% represents the steady state [Ca2+]i in the presence of 40 mM KCl. Forskolin (1 μM) inhibited rhythmic increases in [Ca2+]i and tension without changing the basal [Ca2+]i. EGTA (4 mM) decreased basal [Ca2+]i below the resting level with no further decrease in muscle tension.
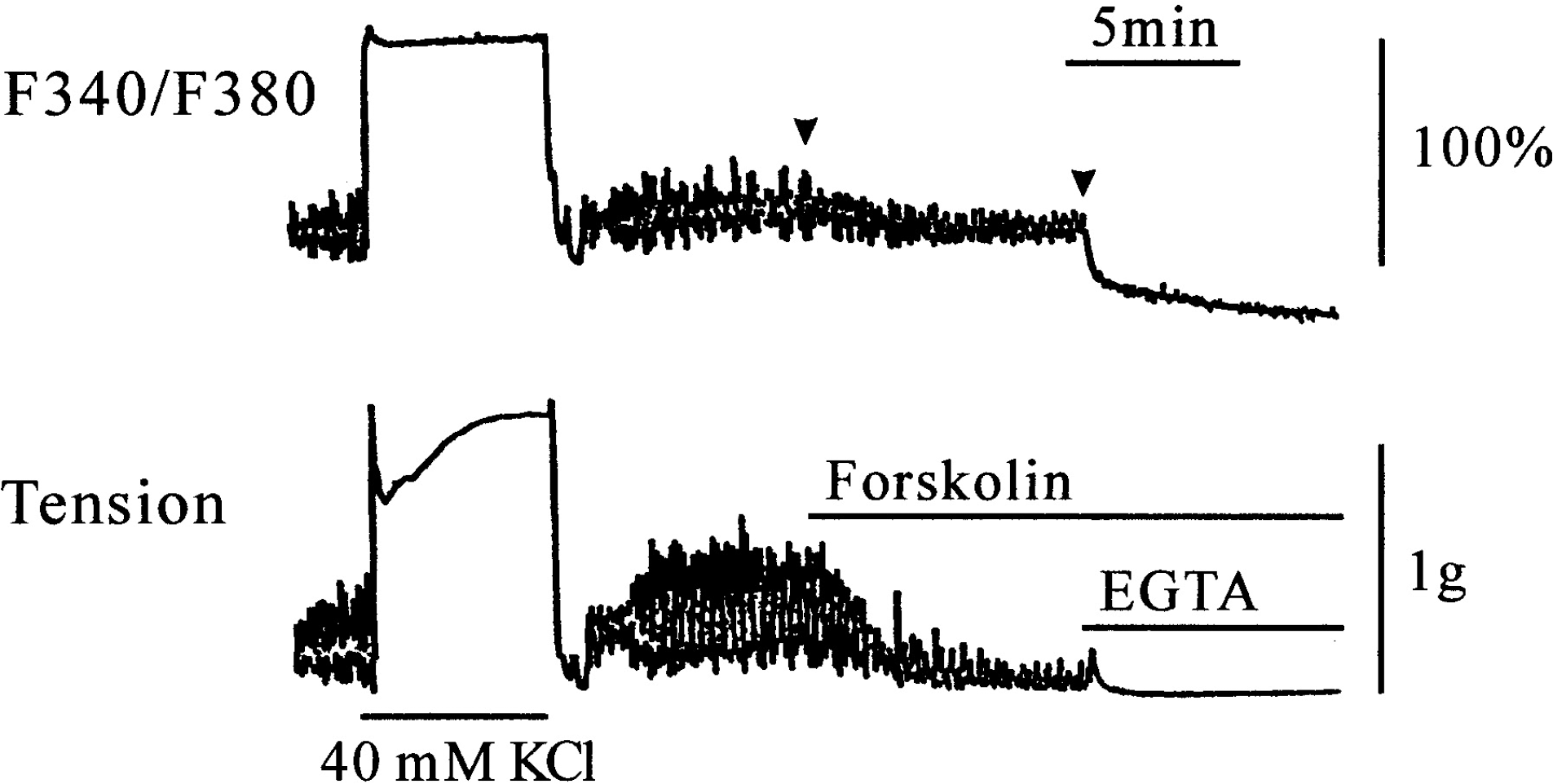
Fig. 3.
Effects of forskolin on 40 mM KCl (A)- and 3 μM histamine (B)-stimulated [Ca2+]i (upper trace) and muscle tension (lower trace). 100% represents the 40 mM KCl- or histamine-induced increases in [Ca2+]i before addition of forskolin. In panel A, after 40 mM KCl-stimulated [Ca2+]i and muscle tension reached a steady state level, 10 μM forskolin and verapamil were sequentially added. Forskolin partially inhibited contraction without changing [Ca2+]i. Verapamil (10 μM), on the other hand, inhibited [Ca2+]i and tension to the resting level. In panel B, when the [Ca2+]i and muscle tension induced by histamine (3 μM) reached a steady state level, 1 μM forskolin and 10 μM verapamil were sequentially added. Forskolin (1 μM) significantly inhibited [Ca2+]i and contractions. Verapamil (10 μM) inhibited [Ca2+]i and tension to the resting level.
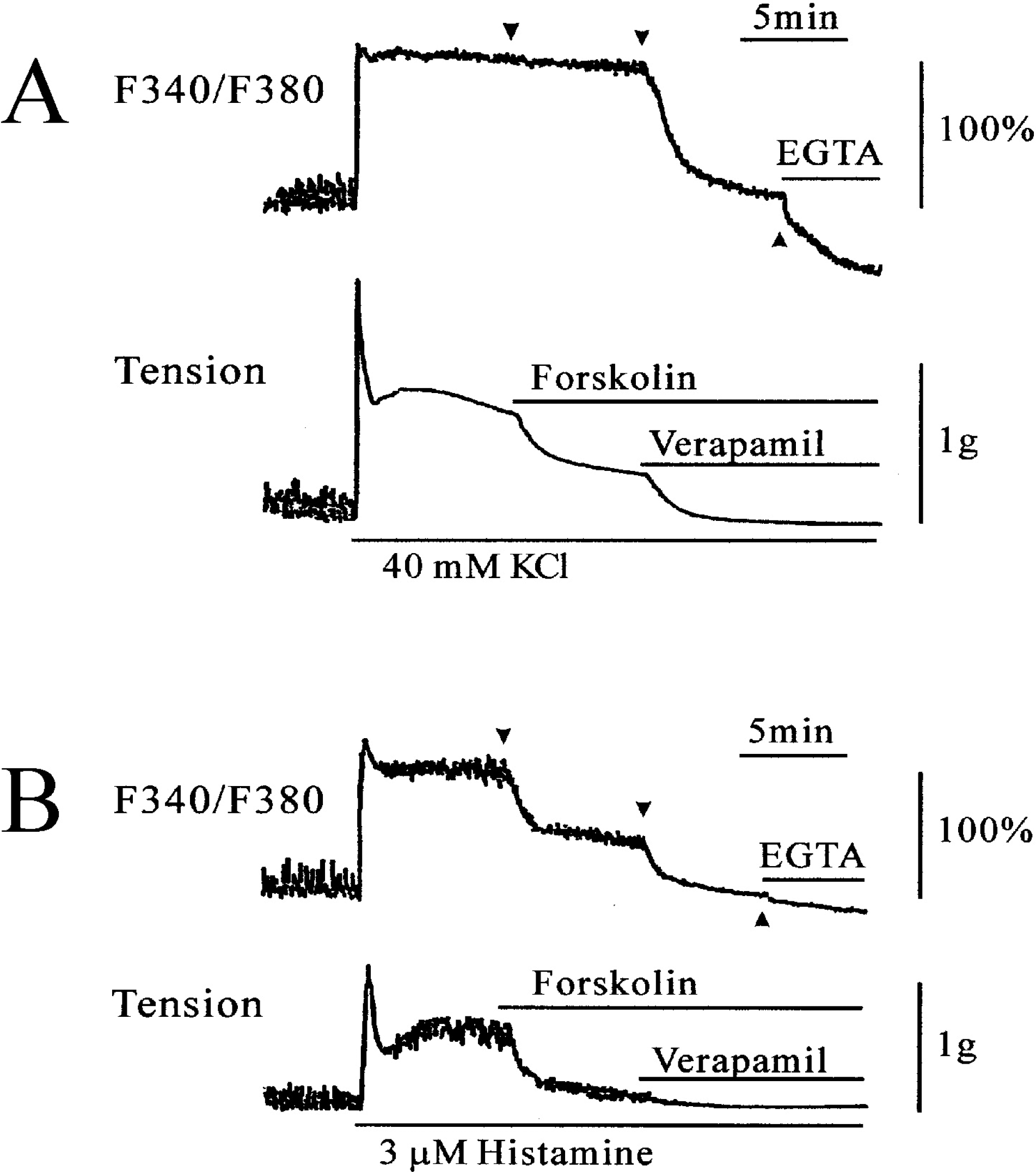
Fig. 4.
Effect of forskolin (10 μM, open circles) on the relationship between [Ca2+]i (abscissa) and muscle tension (ordinate) in the presence various concentrations of KCl (10, 15, 20, 30, and 40 mM) or histamine (0.03, 0.1, 0.3, 1, and 3 μM). 100% represents 40 mM KCl-induced increases in [Ca2+]i and muscle tension measured before cumulative addition of the stimuli. Each point represents the mean of 7~10 experiments, and the SR mean is shown by vertical and horizontal bars.
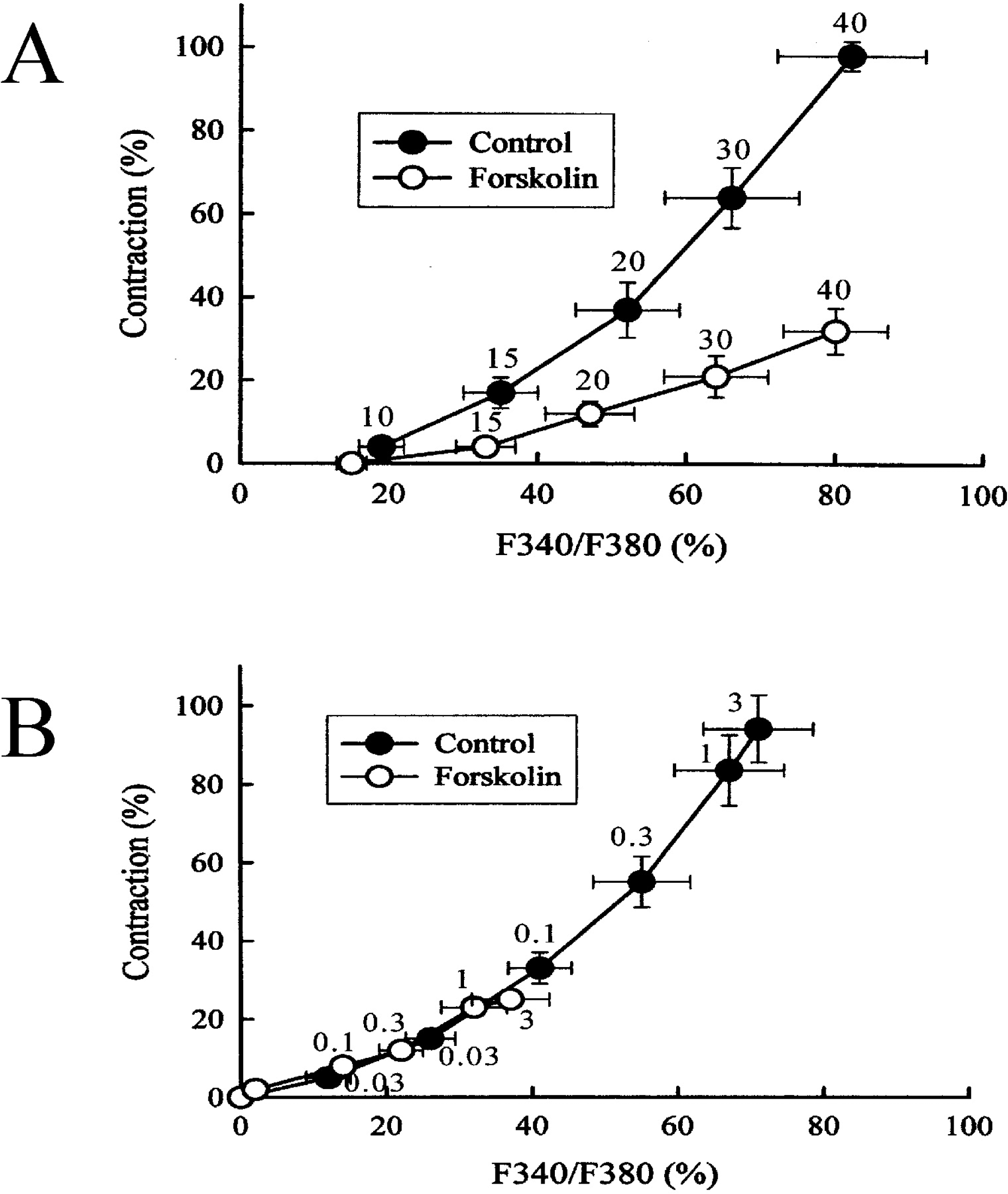
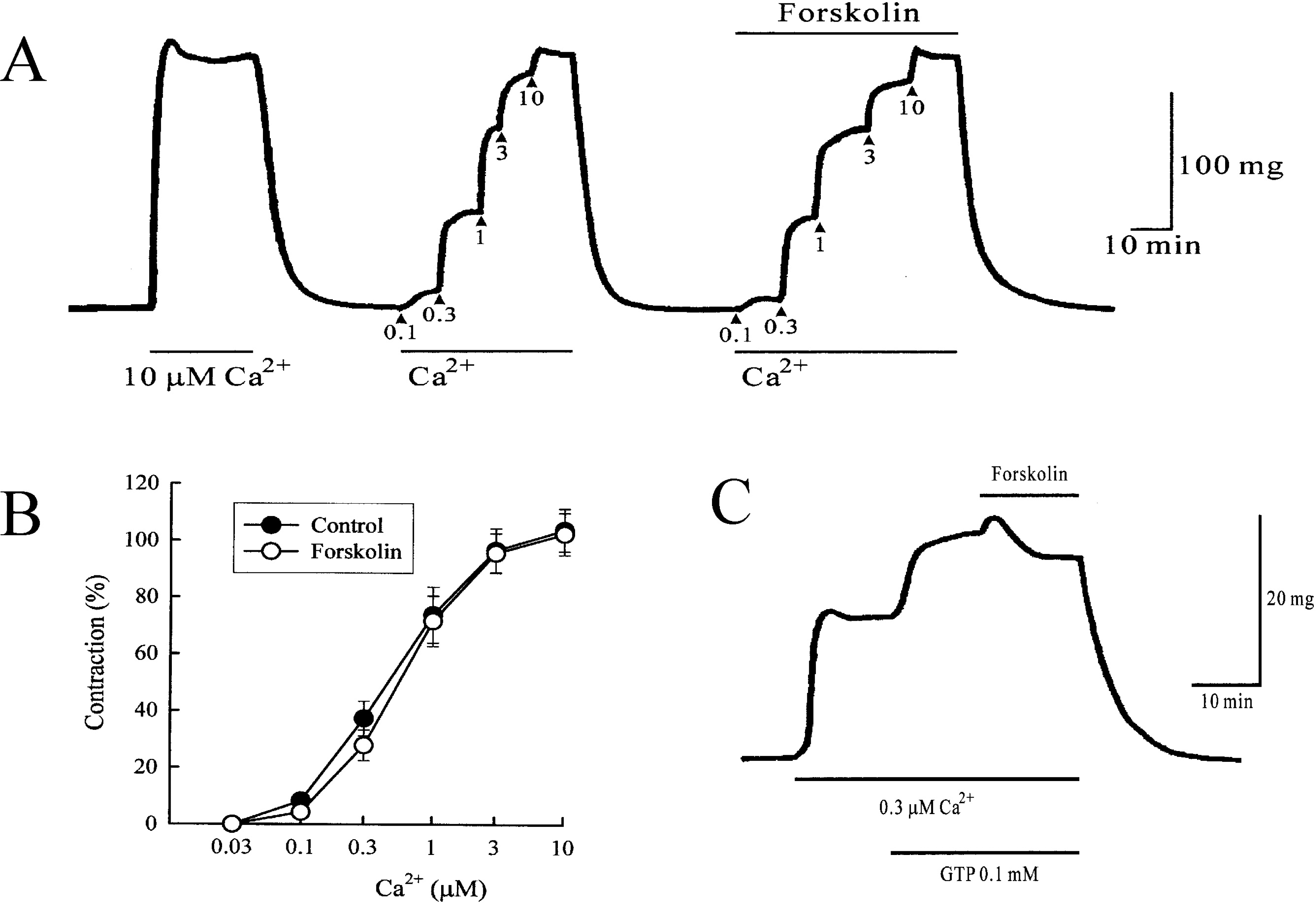
Fig. 5.
(A) Effect of 10 μM forskolin on the contraction evoked by Ca2+ in α-toxin-permeabilized ileum. Ca2+ was cumulatively applied. The experiments were repeated after application of 10 μM forskolin. As a control, 10 μM Ca2+ was applied. (B) The pCa2+-tension relationship observed before (•) and after application of 10 μM forskolin (○). The amplitude of 10 μM Ca2+ was taken as 100%. Each point represents the mean of 6 experiments and the SE mean is shown by a vertical bar. (C) Effects of 10 μM forskolin treated with 0.1 mM GTP. After permebilizing the tissues, 0.3 μM Ca2+ was applied. After the contraction induced by 0.3 μM Ca2+ had reached a steady level, 0.1 mM GTP and forskolin were sequentially applied.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download