Abstract
High concentrations of ATP induce membrane blebbing. However, the underlying mechanism involved in epithelial cells remains unclear. In this study, we investigated the role of the P2×7 receptor (P2×7R) in membrane blebbing using Par C5 cells. We stimulated the cells with 5 mM of ATP for 1 ~ 2 hrs and found the characteristics of membrane blebbing, a hallmark of apoptotic cell death. In addition, 500 μM Bz-ATP, a specific P2×7R agonist, induced membrane blebbing. However, 300 μM of Ox-ATP, a P2×7R antagonist, inhibited ATP-induced membrane blebbing, suggesting that ATP-induced membrane blebbing is mediated by P2×7R. We found that ATP-induced membrane blebbing was mediated by ROCK I activation and MLC phosphorylation, but not by caspase-3. Five mM of ATP evoked a biphasic [Ca2+]i response; a transient [Ca2+]i peak and sustained [Ca2+]i increase secondary to ATP-stimulated Ca2+ influx. These results suggest that P2×7R plays a role in membrane blebbing of the salivary gland epithelial cells.
REFERENCES
Acuna-Castillo C., Coddou C., Bull P., Brito J., Huidobro-Toro JP. Differential role of extracellular histidines in copper, zinc, magnesium and proton modulation of the P2×7 purinergic receptor. J Neurochem. 101:17–26. 2007.
Coleman ML., Sahai EA., Yeo M., Bosch M., Dewar A., Olson MF. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat Cell Biol. 3:339–345. 2001.

Croft DR., Coleman ML., Li S., Robertson D., Sullivan T., Stewart CL., Olson MF. Actin-myosin-based contraction is responsible for apoptotic nuclear disintegration. J Cell Biol. 168:245–255. 2005.

Franke H., Grosche J., Schadlich H., Krugel U., Allgaier C., Illes P. P2X receptor expression on astrocytes in the nucleus accumbens of rats. Neuroscience. 108:421–429. 2001.

Leverrier Y., Ridley AJ. Apoptosis: caspases orchestrate the ROCK ‘n’ bleb. Nat Cell Biol. 3:E91–93. 2001.

Minambres R., Guasch RM., Perez-Arago A., Guerri C. The RhoA/ROCK-I/MLC pathway is involved in the ethanol-induced apoptosis by anoikis in astrocytes. J Cell Sci. 119:271–282. 2006.
Morelli A., Chiozzi P., Chiesa A., Ferrari D., Sanz JM., Falzoni S., Pinton P., Rizzuto R., Olson MF., Di Virgilio F. Extracellular ATP causes ROCK I-dependent bleb formation in P2×7-transfected HEK293 cells. Mol Biol Cell. 14:2655–2664. 2003.
Nobile M., Monaldi I., Alloisio S., Cugnoli C., Ferroni S. ATP-induced, sustained calcium signalling in cultured rat cortical astrocytes: evidence for a non-capacitative, P2×7-like-mediated calcium entry. FEBS Lett. 538:71–76. 2003.
Pfeiffer ZA., Aga M., Prabhu U., Watters JJ., Hall DJ., Bertics PJ. The nucleotide receptor P2×7 mediates actin reorganization and membrane blebbing in RAW 264.7 macrophages via p38 MAP kinase and Rho. J Leukoc Biol. 75:1173–1182. 2004.
Sebbagh M., Renvoize C., Hamelin J., Riche N., Bertoglio J., Breard J. Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat Cell Biol. 3:346–352. 2001.

Sisto M., Lisi S., Castellana D., Scagliusi P., D'Amore M., Caprio S., Scagliusi A., Acquafredda A., Panaro MA., Mitolo V. Autoanti-bodies from Sjogren's syndrome induce activation of both the intrinsic and extrinsic apoptotic pathways in human salivary gland cell line A-253. J Autoimmun. 27:38–49. 2006.
Surprenant A., Rassendren F., Kawashima E., North RA., Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2×7). Science. 272:735–738. 1996.
Zhang X., Zhang M., Laties AM., Mitchell CH. Stimulation of P2×7 receptors elevates Ca2+ and kills retinal ganglion cells. Invest Ophthalmol Vis Sci. 46:2183–2191. 2005.
Fig. 1.
Membrane blebbing induced by ATP. (A) Par C5 cells were treated with 5 mM ATP, 5 mM ATP with 300 μM ox-ATP for 2 hrs, and 5 mM ATP in Ca2+-free solution. The cells were stained with Texas Red-X phalloidin. In addition, 5 mM of ATP for 2 hrs induced several membrane blebs per cell (arrows in the second panel). Membrane blebbing was reduced following pretreatment with 300 μM ox-ATP or 5 mM ATP in a Ca2+-free bath solution (the third and fourth panels). (B) The histogram summarizes the results of the percent of cells demonstrating membrane blebbing following treatment with 1 mM Bz-ATP, 5 mM ATP, 1 mM ADP, or 1 mM UTP. Membrane blebbing was also quantified following ATP or Bz-ATP stimulation following 2 hrs of incubation with 300 μM ox-ATP or in a Ca2+-free solution. The data are the means of five separate experiments. ∗p<0.05 is derived from comparisons with untreated control cells.
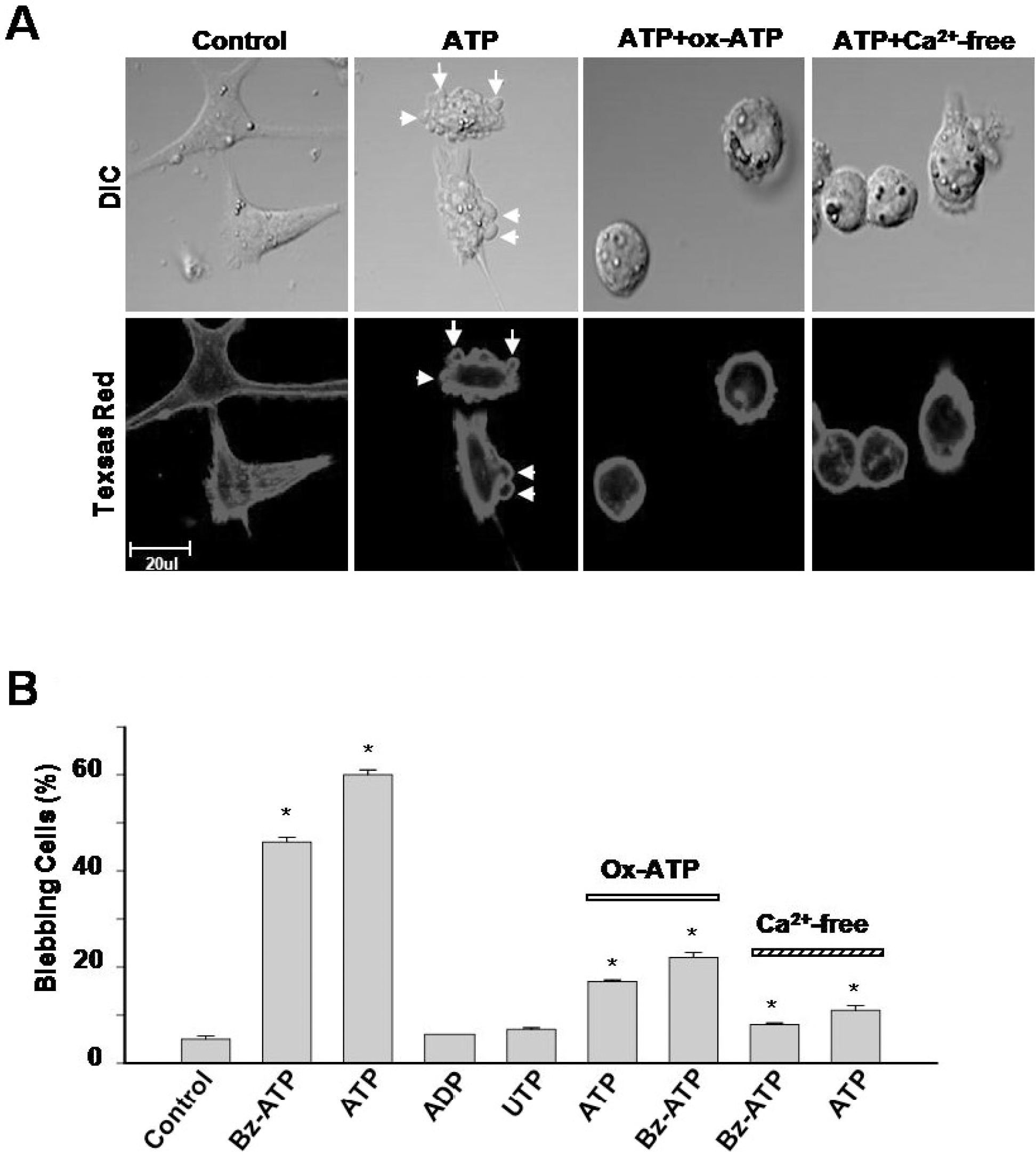
Fig. 2.
ATP induced ROCK I activation and MLC phosphorylation in Par C5 cells. (A) ROCK I cleavage and phosphorylated-MLC (pMLC) from whole cell lysates (50 μg) were resolved by SDS-PAGE and blotted with specific antibodies. The blots were probed with antibodies against ROCK I, pMLC, and MLC (Control). The cells were treated with ATP for up to 2 hrs. (B) ATP-induced ROCK I cleavage and pMLC after pretreatment with 300 μM ox-ATP or in Ca2+-free solution. (C) ATP-induced ROCK I cleavage and pMLC after pretreatment with 300 μM caspase-3 inhibitor (DEVD-fmk) or 10 μM ROCK I inhibitor (Y-27632). (D) The effects of Y-27632 and DEVD-fmk on the percent of cells demonstrating membrane blebbing following 5 mM ATP stimulation. The number of cells containing membrane blebs was determined from a total of 500 cells. The data are means from five separate experiments ∗p<0.05 compared to untreated control cells.
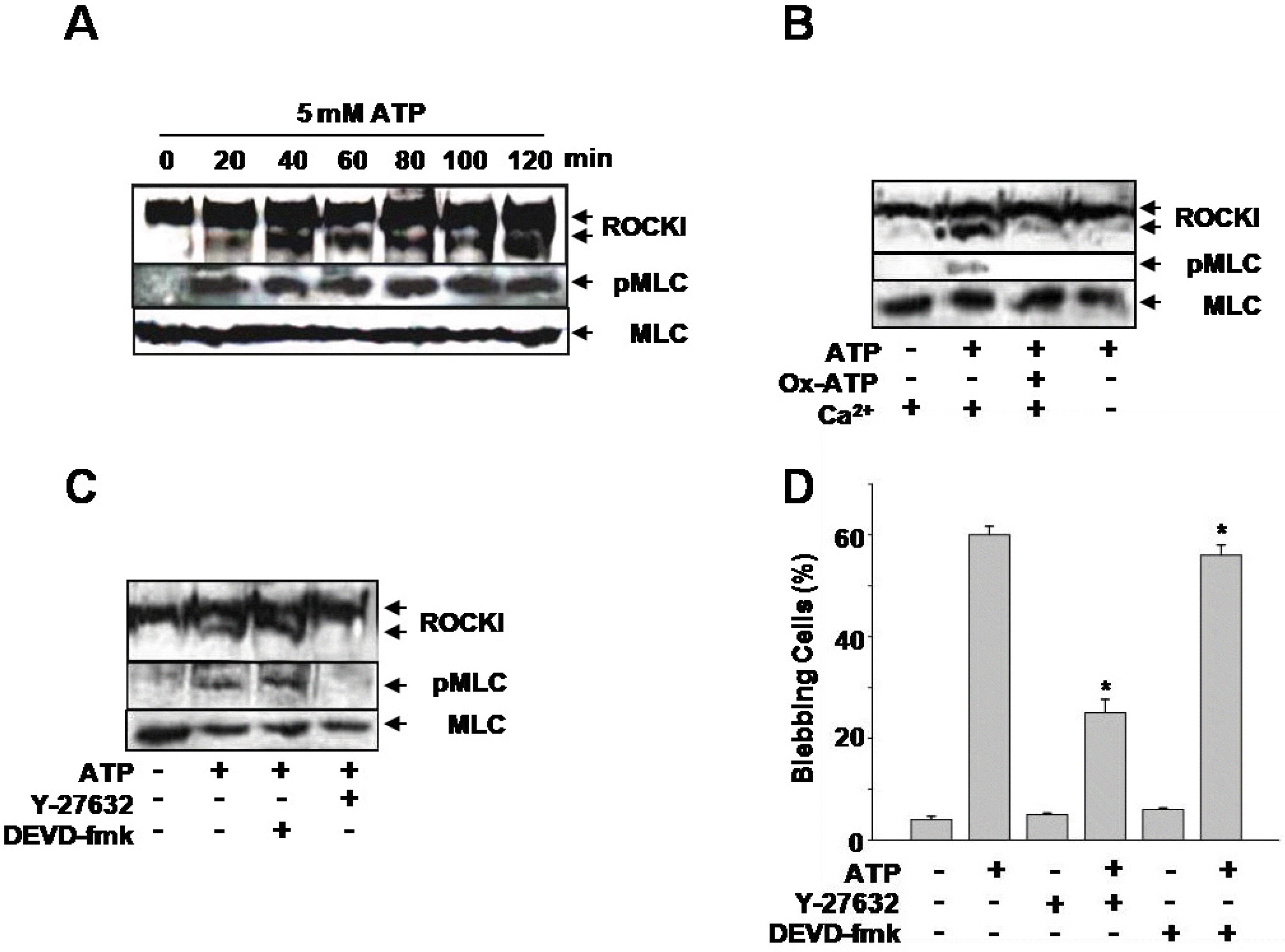
Fig. 3.
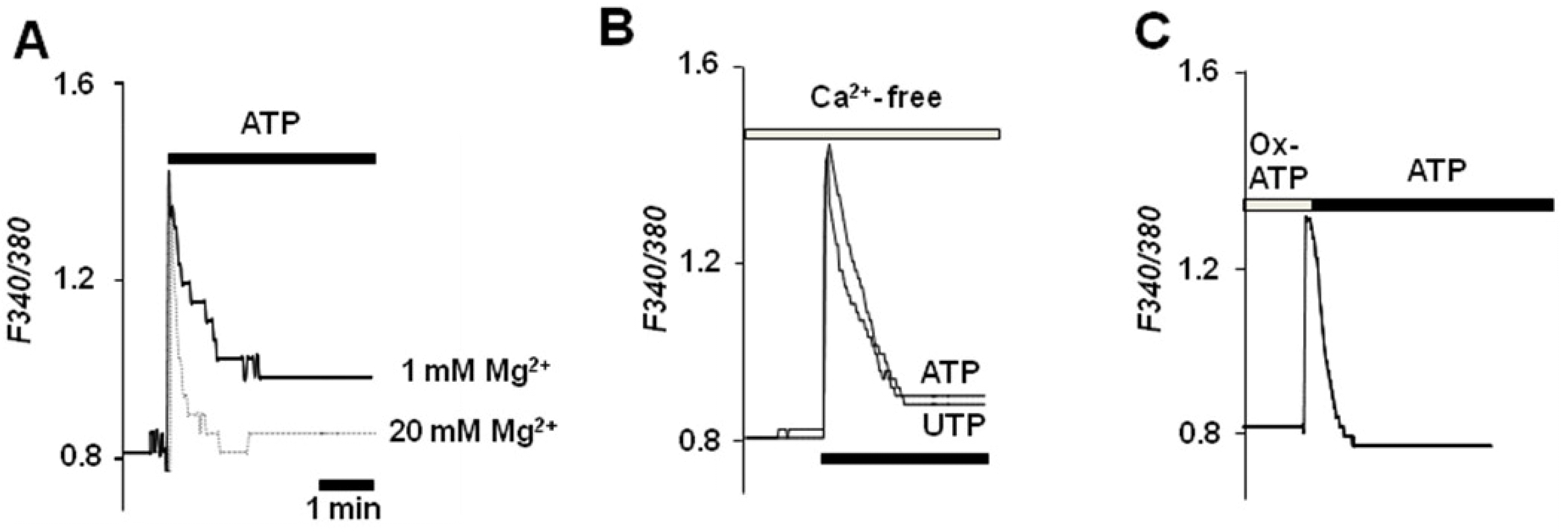
Measurement of cytoplasm free Ca2+ concentrations ([Ca2+]i) mediated by P2×7R activation in fura-2 loaded Par C5 cells. (A) In the presence of 1 mM Mg2+, 5 mM ATP induced a transient and sustained elevation in [Ca2+]i. In addition, 20 mM Mg2+ completely blocked the sustained increased in [Ca2+]. (B) In a Ca2+-free bath solution, the transient peak increase in [Ca2+]i was measured following stimulation with ATP (5 mM) or UTP (1 mM). The sustained increase was absent in the Ca2+-free media. (C) Two hours following preincubation with 300 μM ox-ATP, a peak increase in [Ca2+]i stimulated by 5 mM of ATP was present without evidence of a sustained increase. Each tracing is a typical representative sample of at least 10 separate experiments using different cell preparations.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download