Abstract
Repeated psychostimulants induce electroencephalographic (EEG) changes, which reflect adaptation of the neural substrate related to dopaminergic pathways. To study the role of dopamine receptors in EEG changes, we examined the effect of apomorphine, the dopamine D1 receptor antagonist, SCH-23390, and the D2 receptor antagonist, haloperidol, on EEG in rats. For single and repeated apomorphine treatment groups, the rats received saline or apomorphine for 4 days followed by a 3-day withdrawal period and then apomorphine (2.5 mg/kg, i.p.) challenge after pretreatment with saline, SCH-23390, or haloperidol on the day of the experiment. EEGs from the frontal and parietal cortices were recorded. On the frontal cortex, apomorphine decreased the power of all the frequency bands in the single treatment group, and increased the theta (4.5~8 Hz) and alpha (8~13 Hz) powers in the repeated treatment group. Changes in both groups were reversed to the control values by SCH-23390. On the parietal cortex, single apomorphine treatment decreased the power of some frequency bands, which were reversed by haloperidol but not by SCH-23390. Repeated apomorphine treatment did not produce significant changes in the power profile. These results show that adaptation of dopamine pathways by repeated apomorphine treatment could be identified with EEG changes such as increases in theta and alpha power of the frontal cortex, and this adaptation may occur through changes in the D1 receptor and/or the D2 receptor.
REFERENCES
Binienda Z., Beaudoin MA., Thorn BT., Sadovova N., Skinner RD., Slikker W Jr., Ali SF. Application of electrophysiological method to study interactions between ibogaine and cocaine. Ann N Y Acad Sci. 914:387–393. 2000.

Castner SA., Williams GV. From vice to virtue: insights from sensitization in the nonhuman primate. Prog Neuropsychopharmacol Biol Psychiatry. 31:1572–1592. 2007.

Damianopoulos EN., Carey RJ. Apomorphine sensitization effects: evidence for environmentally contingent behavioral reorganization processes. Pharmacol Biochem Behav. 45:655–663. 1993.
De Vries TJ., Schoffelmeer AN., Binnekade R., Mulder AH., Vanderschuren LJ. Drug-induced reinstatement of heroin- and cocaine-seeking behaviour following long-term extinction is associated with expression of behavioural sensitization. Eur J Neurosci. 10:3565–3571. 1998.

Deroche V., Le Moal M., Piazza PV. Cocaine self-administration increases the incentive motivational properties of the drug in rats. Eur J Neurosci. 11:2731–2736. 1999.

Ferger B., Kropf W., Kuschinsky K. Studies on electroencephalogram (EEG) in rats suggest that moderate doses of cocaine or d-amphetamine activate D1 rather than D2 receptors. Psychopharmacology (Berl). 114:297–308. 1994.
Ferger B., Kuschinsky K. Effects of morphine on EEG in rats and their possible relations to hypo- and hyperkinesia. Psychopharmacology (Berl). 117:200–207. 1995.

Ferger B., Stahl D., Kuschinsky K. Effects of cocaine on the EEG power spectrum of rats are significantly altered after its repeated administration: do they reflect sensitization phenomena? Naunyn Schmiedebergs Arch Pharmacol. 353:545–551. 1996.

Kropf W., Kuschinsky K. Effects of stimulation of dopamine D1 receptors on the cortical EEG in rats: different influences by a blockade of D2 receptors and by an activation of putative dopamine autoreceptors. Neuropharmacology. 32:493–500. 1993.

Kropf W., Kuschinsky K., Krieglstein J. Apomorphine-induced alterations in cortical EEG activity of rats. Involvement of D-1 and D-2 dopamine receptors. Naunyn Schmiedebergs Arch Pharmacol. 340:718–725. 1989.
Kropf W., Kuschinsky K., Krieglstein J. Conditioning of apomorphine effects: simultaneous analysis of the alterations in cortical electroencephalogram and behaviour. Naunyn Schmiedebergs Arch Pharmacol. 343:559–567. 1991.

Kwon JS., Kim KM., Chang SU., Kim CY., Chung TH., Choi BJ., Lee MG. Differential effects of typical and atypical antipsychotics on MK-801 induced EEG changes in rats. Korean J Physiol Pharmacol. 9:17–22. 2005.
Post RM., Rose H. Increasing effects of repetitive cocaine administration in the rat. Nature. 260:731–732. 1976.

Robinson TE., Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 396:157–198. 1986.

Robinson TE., Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 18:247–291. 1993.

Robinson TE., Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 95(Suppl 2):S91–117. 2000.

Segal DS., Mandell AJ. Long-term administration of d-amphetamine: progressive augmentation of motor activity and stereotypy. Pharmacol Biochem Behav. 2:249–255. 1974.

Stahl D., Ferger B., Kuschinsky K. Sensitization to d-amphetamine after its repeated administration: evidence in EEG and behaviour. Naunyn Schmiedebergs Arch Pharmacol. 356:335–340. 1997.

Steketee JD. Neurotransmitter systems of the medial prefrontal cortex: potential role in sensitization to psychostimulants. Brain Res Brain Res Rev. 41:203–228. 2003.

Stewart J., Badiani A. Tolerance and sensitization to the behavioral effects of drugs. Behav Pharmacol. 4:289–312. 1993.

Vanderschuren LJ., Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl). 151:99–120. 2000.

Fig. 1.
EEG band powers of the frontal cortex after apomorphine (APO) treatment in single (S) and repeated (R) apomorphine treatment groups. (A) Band powers in the control state and 25~30 min after APO injection; (B) Band powers in the control state and 55~60 min after APO injection. Each point and bar represent the mean±S.E.M. ∗Significantly different from the control state; #Significantly different between R and S groups (p<0.05 by ANOVA, t-test).
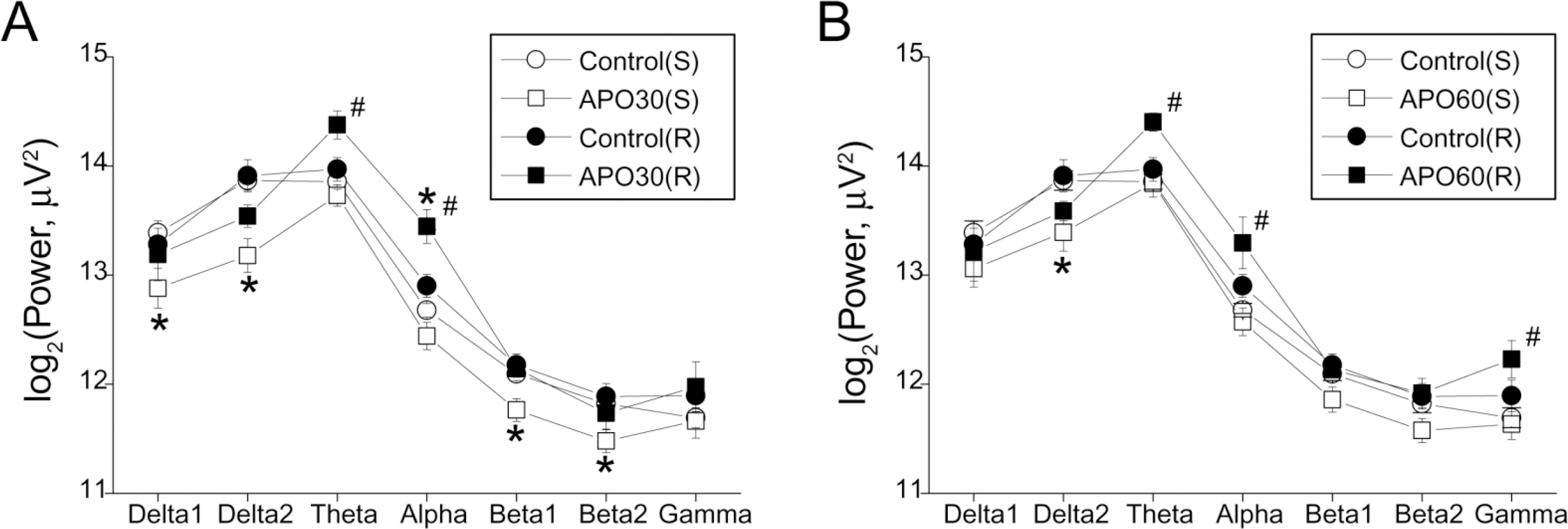
Fig. 2.
EEG band powers of the frontal cortex 25~30 min after apomorphine (APO) injection in single (S, upper panel, A and C) and repeated (R, lower panel, B and D) treatment groups pretreated with: SCH-2390 (SCH, A and B) and haloperidol (HAL, C and D). Each point and bar represent the mean±S.E.M. ∗Significantly different from the control state; #Significantly different from saline (SAL) pretreatment (p<0.05 by ANOVA, t-test).
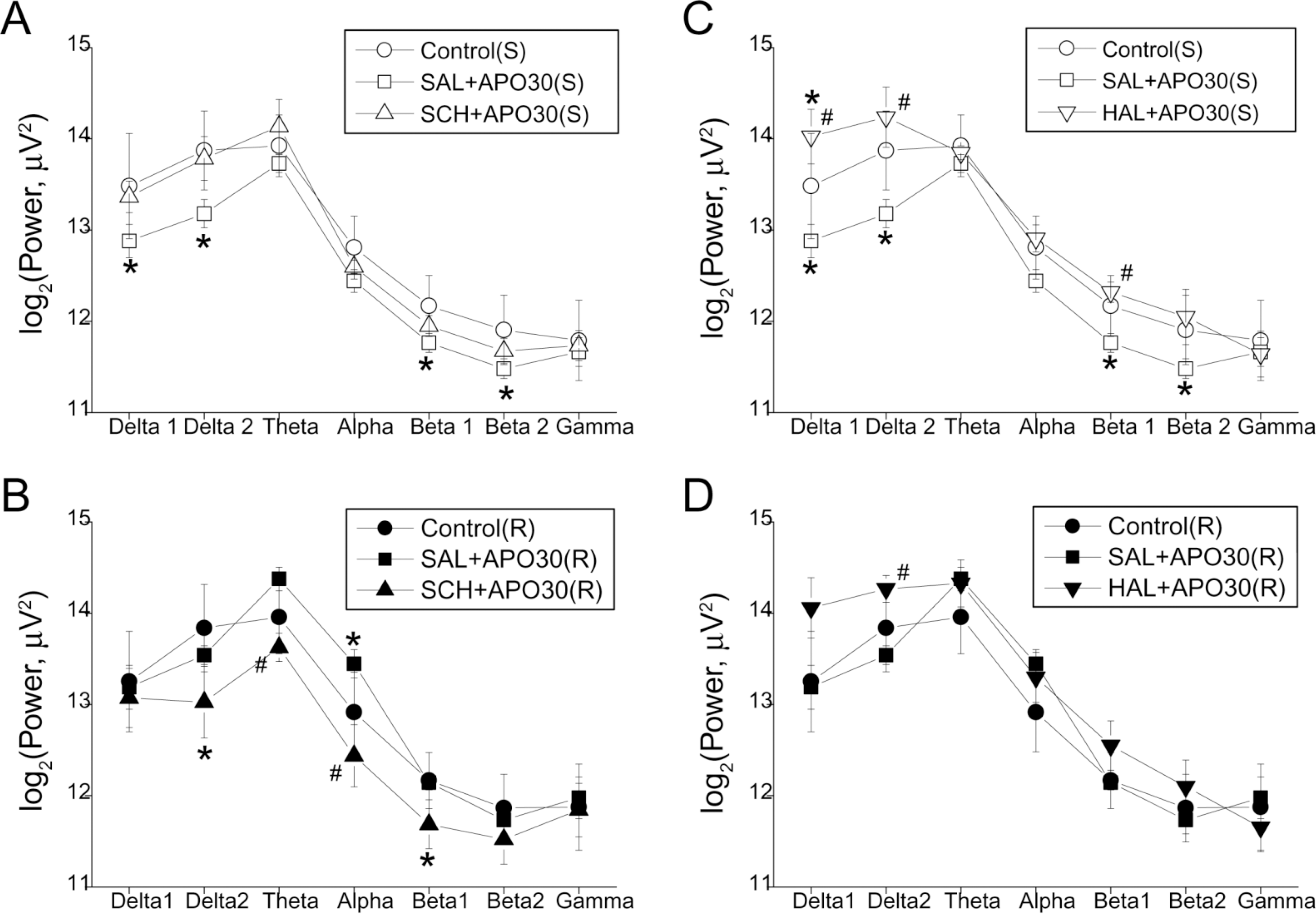
Fig. 3.
EEG band powers of the parietal cortex after apomorphine (APO) treatment in single (S) and repeated (R) apomorphine treatment groups. (A) Band powers in the control state and 25~30 min after APO injection; (B) Band powers in the control state and 55~60 min after APO injection. Each point and bar represent the mean±S.E.M. ∗Significantly different from the control state (p<0.05 by ANOVA, t-test).
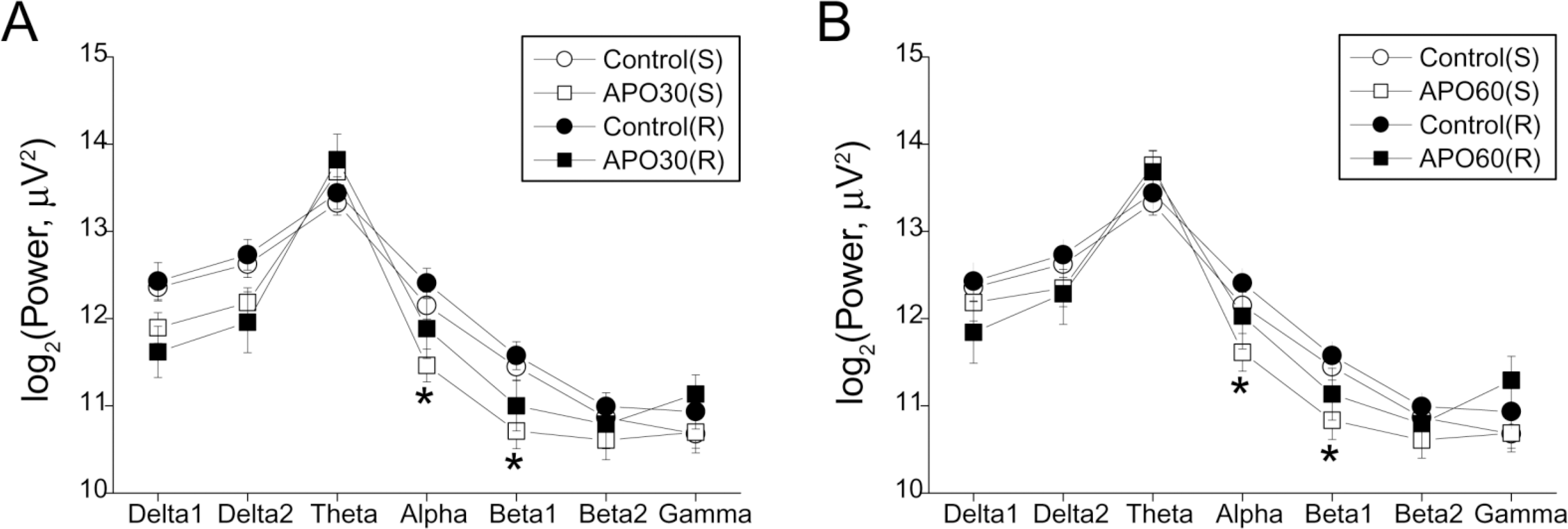
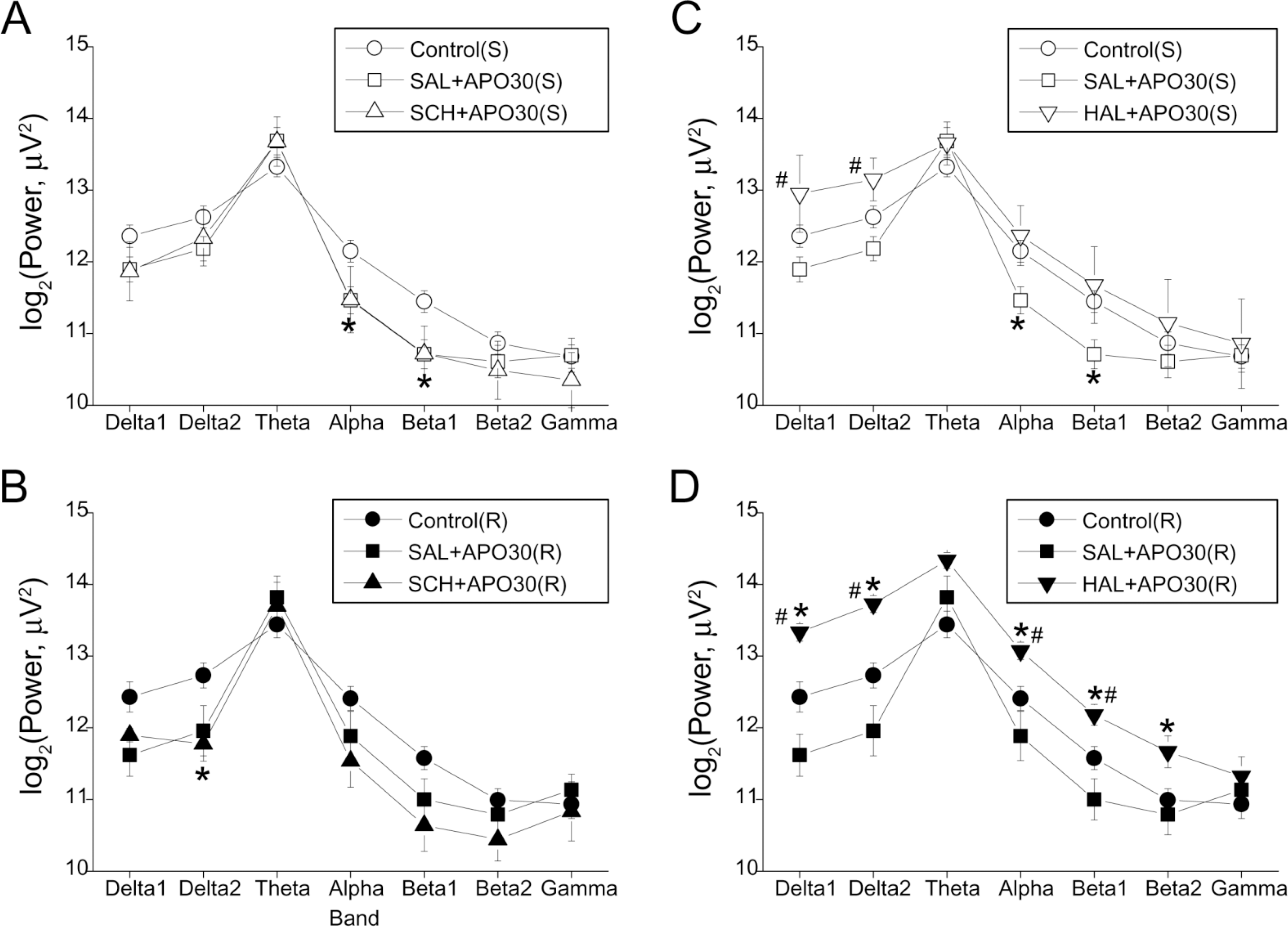
Fig. 4.
EEG band powers of the parietal cortex 25~30 min after apomorphine (APO) injection in single (S, upper panel, A and C) and repeated (R, lower panel, B and D) treatment groups pretreated with: SCH-2390 (SCH, A and B) and haloperidol (HAL, C and D). Each point and bar represent the mean±S.E.M. ∗Significantly different from the control state; #Significantly different from saline (SAL) pretreatment (p<0.05 by ANOVA, t-test).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download