Abstract
Sodium retention is a hallmark of nephrotic syndrome. We investigated whether sodium retention is associated with changes of natriuretic peptide system at different stages (i.e., a sodium retaining stage and a compensatory stage) of nephrotic syndrome. At day 7 after PAN (puromycin aminonucleoside) injection, the urinary excretion of sodium was decreased, along with the development of ascites and positive sodium balance. The plasma and urinary ANP (atrial natriuretic peptide) immunoreactivities were increased. ANP mRNA expression was increased in the heart and kidney, whereas that of NPR (natriuretic peptide receptor)-A and NPR-C mRNA was decreased in the kidney. The expression of NEP was decreased in the kidney. At day 14, urinary excretion of sodium did not differ from the control. The plasma ANP level and heart ANP mRNA expression returned to their control values. The expression of ANP mRNA in the kidney was increased in association with increased urinary ANP immunoreactivities. The expression of NPR-A in the kidney became normal, whereas that of NPR-C kept decreased. The expression of NEP (neutral endopeptidase) remained decreased. These findings suggest that the increased renal ANP synthesis in association with decreased metabolism via NEP and NPR-C may play a compensatory role against the development of sodium retention in nephrotic syndrome. The decreased of NPR-A expression in the kidney may contribute to the ANP resistance at day 7. The subsequent recovery of NPR-A expression may play a role in promoting sodium excretion in later stage (at day 14).
REFERENCES
Deschenes G., Doucet A. Collecting duct Na,K-ATPase activity correlates with urinary sodium excretion in rat nephrotic syndrome. J Am Soc Nephrol. 11:604–615. 2000.
Deschenes G., Gonin S., Zolty E., Cheval L., Rousselot M., Martin PY., Verbavatz JM., Feraille E., Doucet A. Increased synthesis and avp unresponsiveness of Na,K-ATPase in collecting duct from nephrotic rats. J Am Soc Nephrol. 12:2241–2252. 2001.
Drewett JG., Garbers DL. The family of guanylyl cyclase receptors and their ligands. Endocr Rev. 15:135–162. 1994.

Goetz KL., Wang BC., Geer PG., Leadley RJ Jr. Reinhardt HW. Atrial stretch increases sodium excretion independently of release of atrial peptides. Am J Physiol. 250:R946–R950. 1986.
Ichikawa I., Rennke HG., Hoyer JR., Badr KF., Schor N., Troy JL., Lechene CP., Brenner BM. Role for intrarenal mechanisms in the impaired salt excretion of experimental nephritic syndrome. J Clin Invest. 71:91–103. 1983.
Kim SW., Schou UK., Peters CD., de Seigneuxs S., Kwon TH., Knepper MA., Jonassen TE., Frokiaer J., Nielsen S. Increased apical targeting of renal epithelial sodium channel subunits and decreased expression of type 2 11beta-hydroxysteroid dehydrogenase in rats with CCl4-induced decompensated liver cirrhosis. J Am Soc Nephrol. 16:3196–3210. 2005.
Kim SW., Wang W., Nielsen J., Praetorius J., Kwon TH., Knepper MA., Frokiaer J., Nielsen S. Increased expression and apical targeting of renal ENaC subunits in puromycin aminonucleoside-induced nephrotic syndrome in rats. Am J Physiol Renal Physiol. 286:F922–F935. 2004.

Kim SW., Wang W., Sassen MC., Choi KC., Han JS., Knepper MA., Jonassen TE., Frokiaer J., Nielsen S. Biphasic changes of epithelial sodium channel abundance and trafficking in common bile duct ligation-induced liver cirrhosis. Kidney Int. 69:89–98. 2006.

Livak KJ., Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
Parkes DG., Coghlan JP., McDougall JG., Scoggins BA. Long-term hemodynamic actions of atrial natriuretic factor (99-126) in conscious sheep. Am J Physiol. 254:H811–H815. 1988.

Perico N., Delaini F., Lupini C., Benigni A., Galbusera M., Boccardo P., Remuzzi G. Blunted excretory response to atrial natriuretic peptide in experimental nephrosis. Kidney Int. 36:57–64. 1989.

Perico N., Remuzzi G. Edema of the nephrotic syndrome: the role of the atrial natriuretic peptide system. Am J Kidney Dis. 22:355–366. 1993.
Peterson C., Madsen B., Perlman A., Chan AY., Myers BD. Atrial natriuretic peptide and the renal response to hypervolemia in nephrotic humans. Kidney Int. 34:825–831. 1988.

Singer DR., Shore AC., Markandu ND., Buckley MG., Sagnella GA., MacGregor GA. Dissociation between plasma atrial natriuretic peptide levels and urinary sodium excretion after intravenous saline infusion in normal man. Clin Sci. 73:285–289. 1987.

Valentin JP., Qiu C., Muldowney WP., Ying WZ., Gardner DG., Humphreys NH. Cellular basis for blunted volume expansion natriuresis in experimental nephrotic syndrome. J Clin Invest. 90:1302–1312. 1992.

Fig. 1.
The concentration of ANP in plasma and urine. columns show ANP concentration in control and PAN-treated rats. At day 7, the plasma and urinary concentrations of ANP were significantly increased. At day 14, urinary ANP excretion was increased in PAN rats compared with controls, while the plasma ANP level was comparable between two groups (mean±SEM, n=8 each). ∗p<0.05 vs. control.
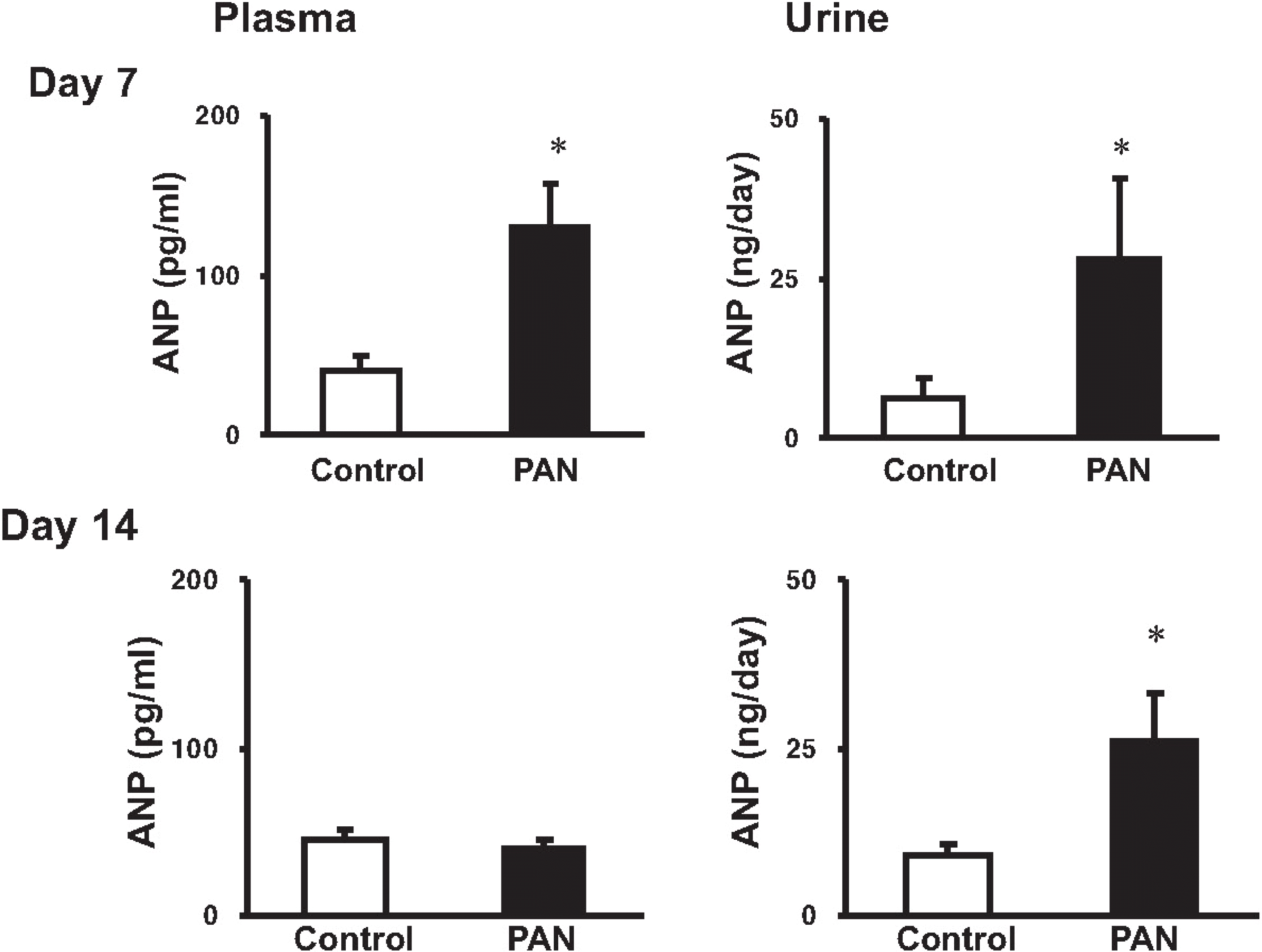
Fig. 2.
Expression of ANP mRNA in the heart and kidney. columns show real-time PCR data representing control and PAN-treated rats. At day 7, the mRNA expression of cardiac ANP was significantly increased in the PAN-induced nephrotic syndrome. At day 14, the mRNA expression of ANP was not changed. At day 7 and 14, the mRNA expression of renal ANP was significantly increased in the PAN-induced nephrotic syndrome compared with controls (mean±SEM, n=8 each). ∗p<0.05 vs. control.
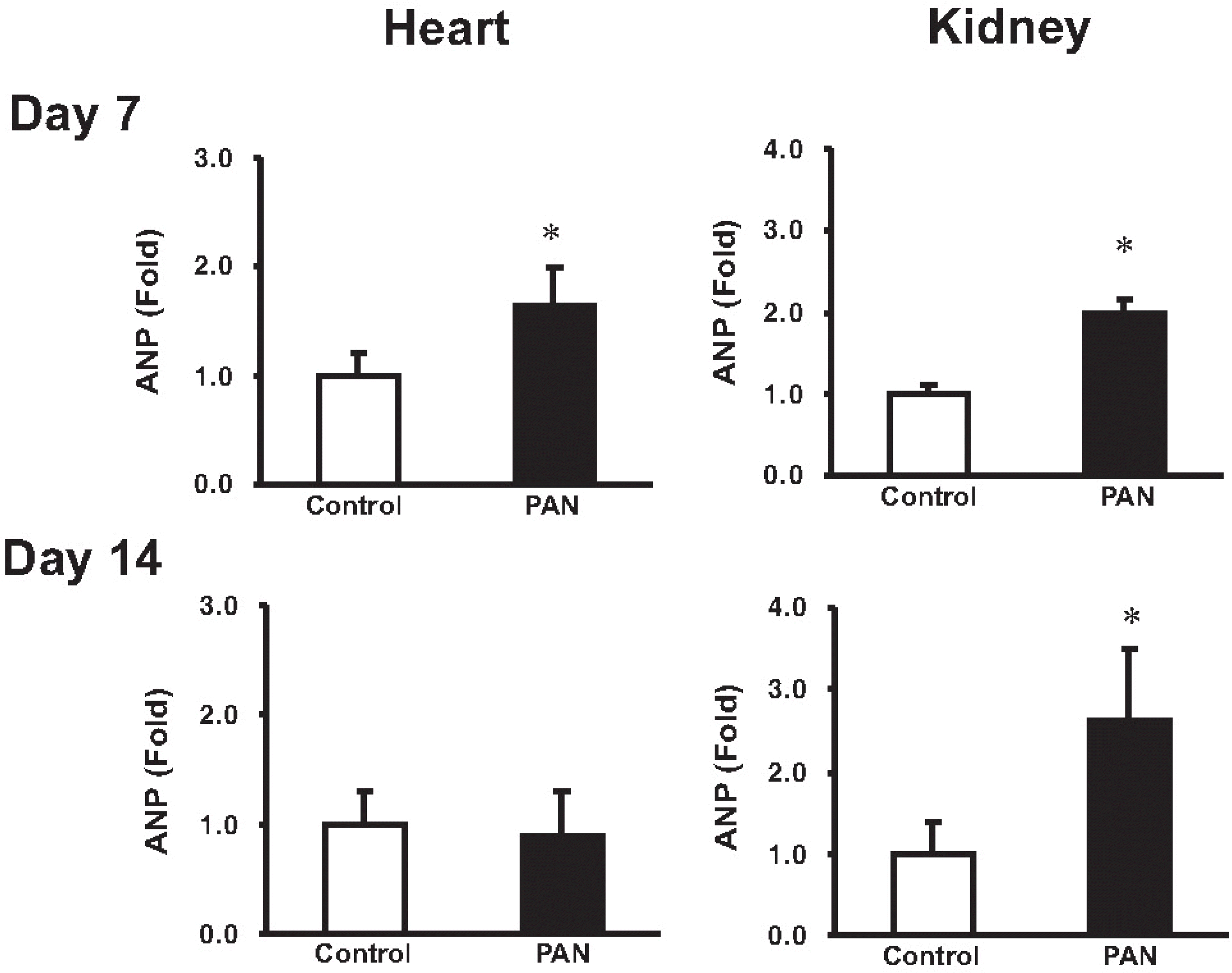
Fig. 3.
Expression of NPR-A and NPR-C mRNA in the kidney. columns show real-time PCR data representing control and PAN-treated rats. At day 7, the mRNA expression of NPR-A and NPR-C expression was decreased. At day 14, the mRNA expression of NPR-C was decreased compared with control, while the NPR-A expression was not changed (mean±SEM, n=8 each). ∗p<0.05 vs. control.
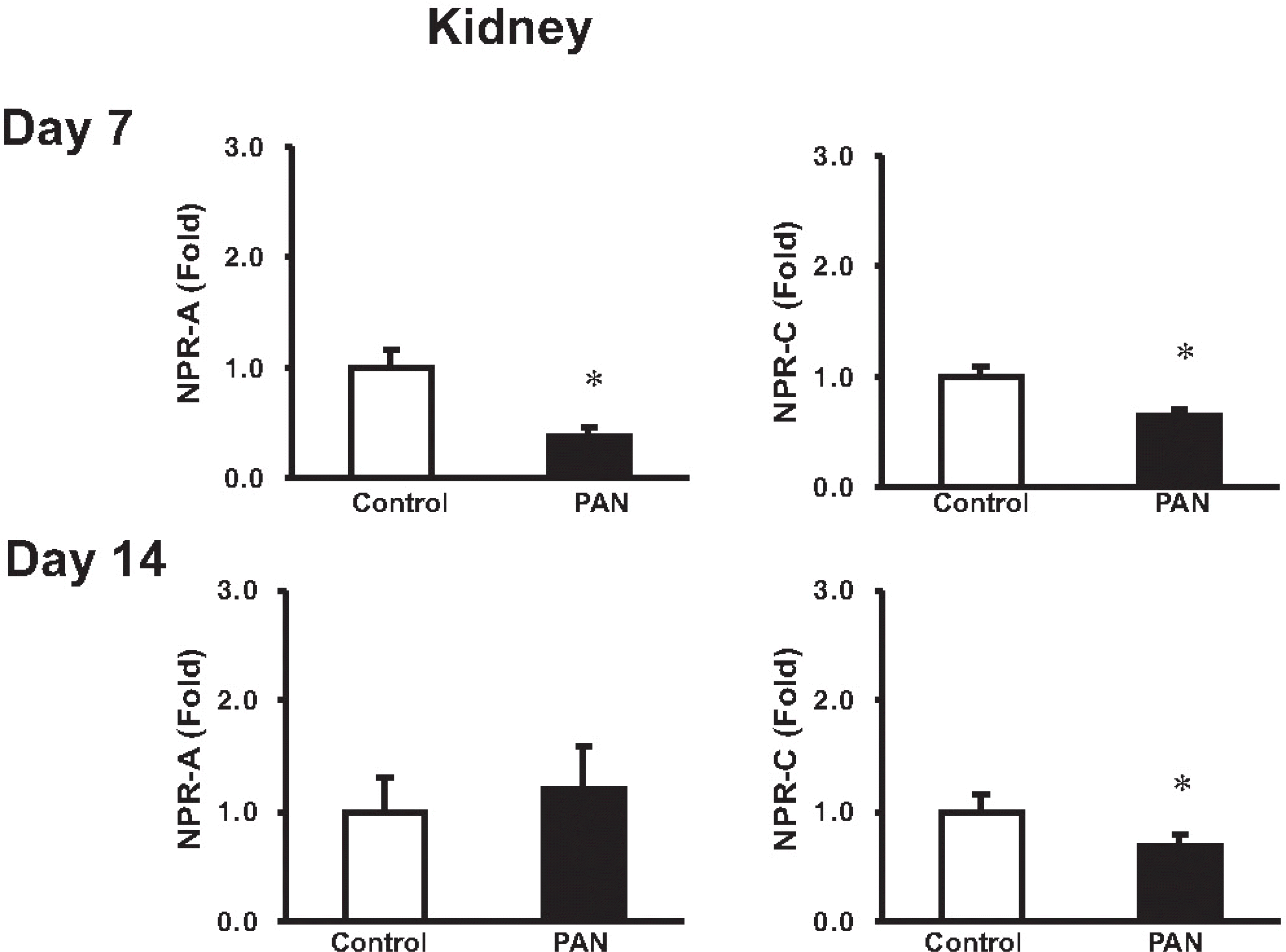
Fig. 4.
(A) Immunoblotting of NEP was decreased in PAN-treated rats compared with control. (B) Immunoperoxidase microscopy of NEP labeling was associated with apical plasma membrane of the proximal tubule in control rats. Immunoperoxidase microscopy demonstrated decreased NEP immunolabeling in the proximal tubule in PAN-treated rats (mean±SEM, n=8 each). ∗p<0.05 vs. control. Magnification: ×200.

Table 1.
Primers used in the polymerase chain reaction
Table 2.
Changes in renal function
| Day 7 | Day 14 | |||
|---|---|---|---|---|
| Control (n=8) | PAN (n=8) | Control (n=8) | PAN (n=8) | |
| Body weight (g) | 178±8 | 224±8∗ | 201±3 | 177±22∗ |
| Pcr (mg/dL) | 0.20±0.05 | 0.40±0.12∗ | 0.29±0.03 | 0.16±0.05∗ |
| Ccr (ml/min) | 1.52±0.3 | 0.78±0.4∗ | 1.32±0.12 | 1.89±0.63∗ |
| UNaV (mEq/day) | 2.78±0.28 | 0.60±0.13∗ | 2.99±0.29 | 3.69±0.99 |
| FENa (%) | 0.9±0.2 | 0.4±0.1∗ | 1.14±0.16 | 1.01±0.29 |
| Na balance (mmol/day) | −1.42±0.27 | 0.30±0.12∗ | −1.64±0.29 | −2.34±0.98 |
Values are expressed as mean±SEM. These values were obtainedat the last day of experiments. P-Cr, plasma creatinine; Ccr, creatinine clearance; UNaV, rate of urinary sodium excretion; FENa, fractional excretion of sodium into urine; Na balance, the difference between dietary sodium intake and urinary sodium excretion.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download