Abstract
The properties of voltage dependent Ca2+ current (VDCC) were investigated in interstitial cells of Cajal (ICC) distributed in the myenteric layer (ICC-MY) of guinea-pig antrum. In tissue, ICC-MY showed c-Kit positive reactions and produced driving potentials with the amplitude and frequency of about 62 mV and 2 times min−1, respectively, in the presence of 1 μM nifedipine. Single ICC-MY isolated by enzyme treatment also showed c-Kit immunohistochemical reactivity. These cells were also identified by generation of spontaneous inward current under K+-rich pipette solution. The voltage clamp experiments revealed the amplitude of – 329 pA inward current at irregular frequency. With Cs+-rich pipette solution at Vh=−80 mV, ICC-MY produced voltage-dependent inward currents (VDIC), and nifedipine (1 μM) blocked VDIC. Therefore, we successfully isolated c-Kit positive single ICC from guinea-pig stomach, and found that ICC-MY potently produced dihydropiridine sensitive L-type voltage-dependent Ca2+ currents (VDCCL).
REFERENCES
Beckett EAH., Ro S., Bayquinov Y., Sanders KM., Ward SM. Kit signaling is essential for development and maintenance of interstitial cells of Cajal and electrical rhythmicity in the embryonic and gastrointestinal tract. Dev Dyn. 236:60–72. 2007.
Cayabyab FS., DeBruin H., Jimenez M., Daniel EE. Ca2+ role in myogenic and neurogenic activities of canine ileum circular muscle. Am J Physiol. 271:G1053–G1066. 1996.
Dickens EJ., Edward FR., Hirst GDS. Selective knockout of intramuscular interstitial cell reveals their role in the generation of slow waves in mouse stomach. J Physiol. 531:827–833. 2001.
Goto K., Matsuoka S., Noma A. Two types of spontaneous depolarizations in the interstitial cells freshly prepared from the murine small intestine. J Physiol. 559:409–420. 2004.

Dickens EJ., Hirst GDS., Tomita T. Identification of rhythmically active cells in guinea-pig stomach. J Physiol. 514:515–531. 1999.

Hamil OP., Marty A., Neher E., Sakamnn B., Sigworth FJ. Improved patch-clamp technique for high resolution current from cells ands cell-free membrane patches. Pflügers Arch. 391:85–100. 1981.
Hennig GW., Hirst GD., Park KJ., Smith CB., Sanders KM., Ward SM., Smith TK. Propagation of pacemaker activity in the guinea-pig antrum. J Physiol. 556:585–599. 2004.

Hirst GD., Edward FR. Generation of slow waves in the antral region of guinea-pig stomach – a stochastic process. J Physiol. 535:165–180. 2001.

Hirst GDS., Ward SM. Interstitial cells: involvement in rhythmicity and neural control of gut smooth muscle. J Physiol. 550:337–346. 2003.

Huizinga JD., Thuneberg L., Kluppel M., Malysz J., Mikkelsen HB., Bernstein A. W/Kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 373:347–349. 1995.
Kim YC., Koh SD., Sanders KM. Voltage-dependent inward currents of interstitial cells of Cajal from murine colon and small intestine. J Physiol. 541:797–810. 2002.
Kito Y., Suzuki H. Pacemaker frequency is increased by sodium nitroprusside in the guinea pig gastric antrum. J Physiol. 546:191–205. 2003.

Kito Y., Ward SM., Sanders KM. Pacemaker potential generated by interstitial cells of Cajal in the murine intestine. J Physiol. 288:C710–C720. 2005.
Klüppel M., Huizinga JD., Malysz J., Bernstein A. Developmental origin and Kit- dependent development of the interstitial cells of Cajal in the mammalian small intestine. Dev Dyn. 211:60–71. 1998.
Koh SD., Jun JY., Kim TW., Sanders KM. A Ca2+-inhibited non-selective cation conductance contributes to pacemaker currents in mouse interstitial cells of Cajal. J Physiol. 540:803–814. 2002.
Koh SD., Monaghhan K., Ro S., Mason HS., Kenyon JL., Sanders KM. Novel voltage-dependent non-selective cation conductance in murine colonic myocytes. J Physiol. 533:341–355. 2001.

Koh SD., Sanders KM., Ward SM. Spontaneous electrical rhythmicity in cultured interstitial cells of Cajal from the murine small intestine. J Physiol. 513:203–213. 1998.

Komuro T., Tokui K., Zhou DS. Identification of interstitial cells of Cajal. Histol Histopathol. 11:769–786. 1996.
Kubota M., Kanda E., Ida K., Sakakihara Y., Hayashi M. Severe gastrointestinal dysmotility in a patient with congenital myopathy: causal relationship to decrease of interstitial cells of Cajal. Brain Develop. 27:447–450. 2005.

Lammers WJ., Stephen B., Adeghate E., Ponery S., Pozzan O. The slow wave does not propagate across the gastroduodenal junction in the isolated feline preparation. Neurogastroenterol Motil. 10:339–349. 1998.

Langton P., Ward SM., Carl A., Norell MA., Sanders KM. Spontaneous electrical activity of interstitial cells of Cajal isolated from canine proximal colon. Proc Natl Acad Sci. 86:7280–7284. 1989.

Lee HK., Sanders KM. Comparision of ionic currents from interstitial cells and smooth muscle cells of canine colon. J Physiol. 460:135–152. 1993.
Lee JI., Park HJ., Kamm MA., Talbot IC. Decreased density of interstitial cells of Cajal and neuronal cells in patients with slow-transit constipation and acquired megacolon. J Gastroenterol Hepatol. 20:1292–1298. 2005.

Malysz J., Richaedson D., Farraway L., Christen MOM., Huizinga JD. Generaion of slow wave type action potentials in the mouse small intestine involves a non-L-type calsium channel. Can J Physiol Pharmacol. 73:1502–1511. 1995.
Ördög T., Redelman D., Miller LJ., Horváth VJ., Zhong Q., Almeida-Porada G., Zanjani ED., Horowitz B., Sanders KM. Purification of interstitial cells of Cajal by fluorescence-activated cell sorting. Am J Physiol. 286:C448–C456. 2004.

Oue T., Puri P. Smooth muscle cell hypertrophy versus hyperplasia in infantile hypertrophic pyloric stenosis. Pediatric Res. 45:853–857. 1999.

Pucovsky V., Moss Ray., Bolton TB. Non-contractile cells with thin processes resembling interstitial cells of Cajal found in the wall of guinea-pig mesenteric arteries. J Physiol. 552:119–133. 2003.
Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 111:492–515. 1996.

Sergeant GP., Hollywood MA., McCloskey KD., Thornbury KD., McHale NG. Specialized pacemaking cells in the rabbit urethra. J Physiol. 526:359–366. 2000.
Thomsen L., Robinson TL., Lee JCF., Farraway LA., Hughes MJG., Andrew DW., Huizinga JD. Interstitial cells of Cajal generate a rhythmic pacemaker current. Nat Med. 4:848–851. 1998.

Torihashi S., Ward SM., Nishikawa S., Kobayashi S., Sanders KM. c-Kit-dependent development of interstitial cells and electrical activity in the murine gastrointestinal tract. Cell Tissue Res. 280:97–111. 1995.
Torihashi S., Ward SM., Sanders KM. Development of c-Kit-positive cells and the onset of electrical rhythmicity in murine small intestine. Gastroenterology. 112:144–155. 1997.
Wang B., Kunze WA., Zhu Y., Huizinga JD. In situ recording from gut pacemaker cells. Pflügers Arch. 457:243–251. 2008.

Wang XY., Lammers WJEP., Bercik P., Huizinga JD. Lack of pyloric interstitial cells of Cajal explains distinct peristalsis motor patterns in stomach and small intestine. Am J Physiol. 289:G539–G549. 2005.
Wang XY., Paterson C., Huizinga JD. Cholineregic and nitrergic innervation of ICC-DMP and ICC-IM in the human small intestine. Neurogastroenterol Motil. 15:531–543. 2003.
Ward SM., Burns AJ., Torihashi S., Sanders KM. Mutation of the proto-oncogene c-Kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol. 480:91–97. 1994.
Ward SM., Dixon RE., Faoite A., Sanders KM. Voltage-dependent calcium entry underlies propagation of slow waves in canine gastric antrum. J Physiol. 561:793–810. 2004.

Ward SM., Sanders KM. Upstroke potential of electrical slow waves in canine colonic smooth muscle due to nifedipine-resistant calcium current. J Physiol. 455:321–337. 1992.
Xu WX., Kim SJ., Kim SJ., So I., Kang TM., Rhee JC., Kim KW. Effect of stretch on calcium channel currents recorded from the antral myocytes of guinea-pig stomach. Pflügers Arch. 432:159–164. 1996.
Fig. 1.
Identification of ICC in the antral region of guinea-pig stomach by c-Kit immunohistochemical activity. (A) reveals the network of ICC-MY in myenteric border of guinea-pig gastric antrum. In (A), arrow head and arrow indicate cell body and processes from ICC-MY, respectively. (B) ICC-IM in circular muscle layers is shown. Scale bars in (A) and (B) are 40 μM.
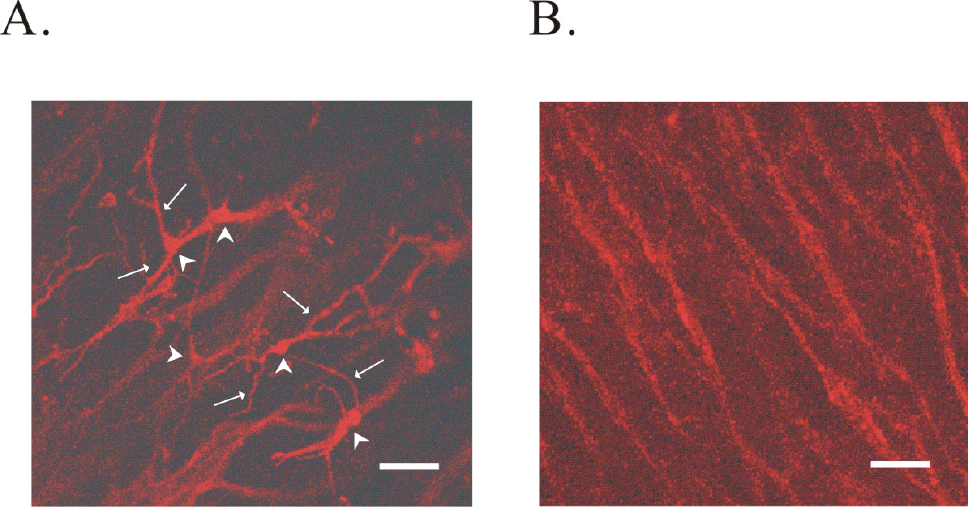
Fig. 2.
Pacemaker potential of ICC-MY in myenteric area of guinea-pig gastric antrum. Spontaneous electrical activity of ICC-MY in guinea-pig antrum was recorded in the presence of 1 μM nifedipine. (A) and (B) Driving potential from ICC-MY was recorded in the presence of 1 μM nifedipine. (B) Expended event of pacemaker potential was displayed.
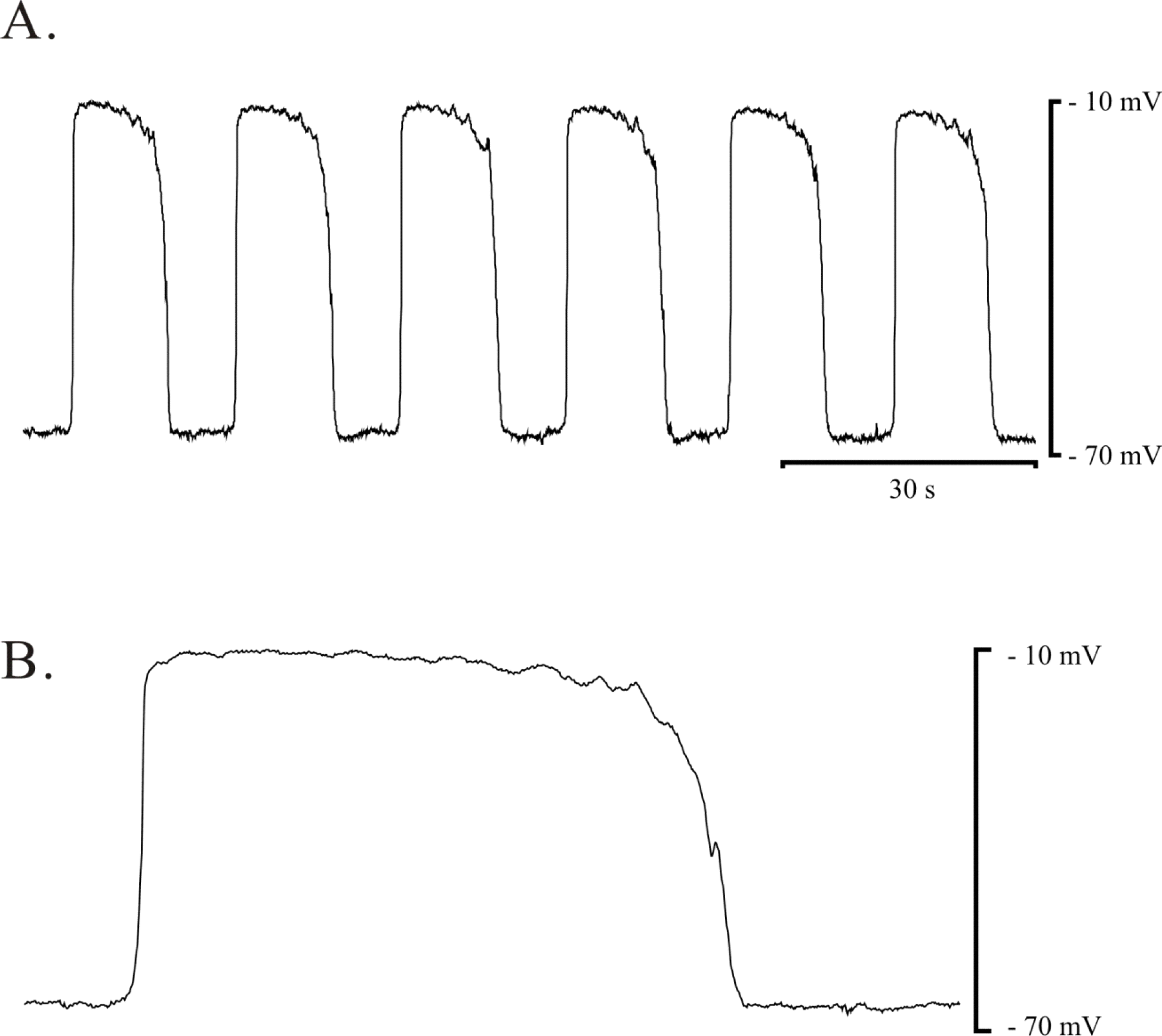
Fig. 3.
Identification of freshly isolated single ICC-MY. Single ICC was isolated by enzyme treatment from myenteric border of gastric antrum in guinea-pig. (A) ICC-MY with many processes from myenteric border of guinea-pig gastric antrum is shown. ICC expressed c-Kit activity (B). (B) shows phenotype (left; arrow head) and c-Kit immunohistochemical reactivity (right; arrow head) of ICC-MY. Lower figures of (B) represent magnification of the upper (B). However, gastric smooth muscle cells (arrows) did not express c-Kit activity (right upper panel). Scale bars in (A) and (B) indicate 40 μM. Note: The left side of panel (B) is phase contrast image from guinea-pig gastric ICC. The right side of panel (B) is c-Kit immunoreactivity image.
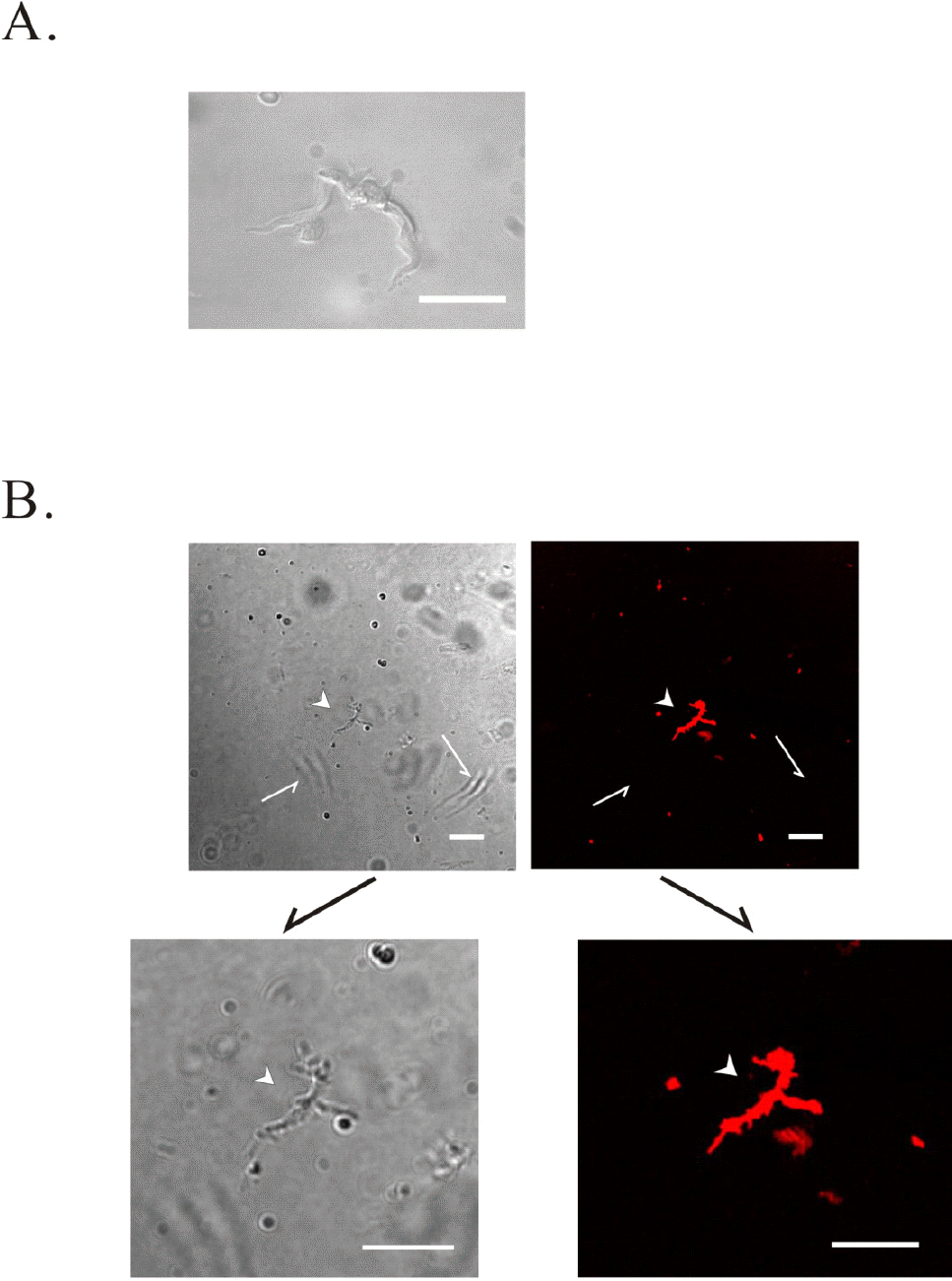
Fig. 4.
Spontaneous inward current recorded from freshly isolated single gastric ICC from guinea-pig antrum. Using the conventional whole cell patch-clamp technique, we observed spontaneous inward current in freshly isolated single ICC from guinea-pig gastric antrum. (A) Spontaneous inward current was recorded irregularly under K+-rich pipette solution. (B) and (C) ICC showed spontaneous inward current in the presence (C) and absence (B) of 1 μM nifedipine under Cs+-rich pipette solution. The data from (B) and (C) are summarized in (D).
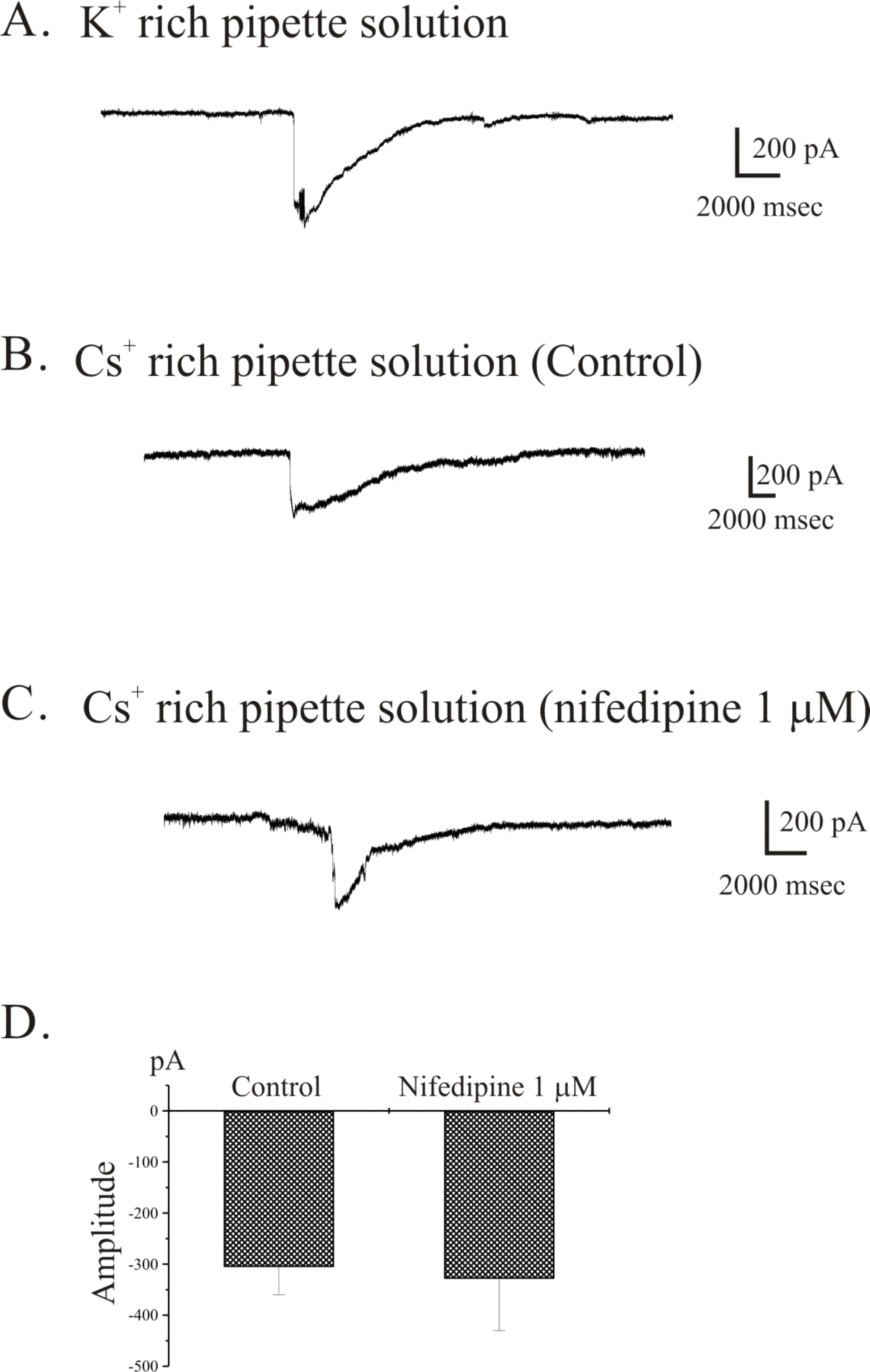
Fig. 5.
Voltage-dependent Ca2+ currents (VDCC) in freshly isolated single ICC from guinea-pig antrum. At a holding potential of −80 mV, spontaneous inward current was recorded in freshly isolated single ICC as shown in Fig. 4B. From these cells, voltage-dependent inward current (VDIC) was recorded by step pulses. (A) Currents were elicited by 20 mV depolarizing step pulses from a holding potential of −80 mV to +40 mV (voltage protocol is shown in inset). (B) Currents were elicited by voltage steps from a holding potential of −80 mV to 0 mV. The peak amplitudes generated were compared before and after nifedipine (1 μM) treatment. (C) Current-voltage relationships of before (filled circles) and after (open circles) treatment of 1 μM nifedipine are shown. (D) The blocking effect of nifedipine on VDIC is summarized.
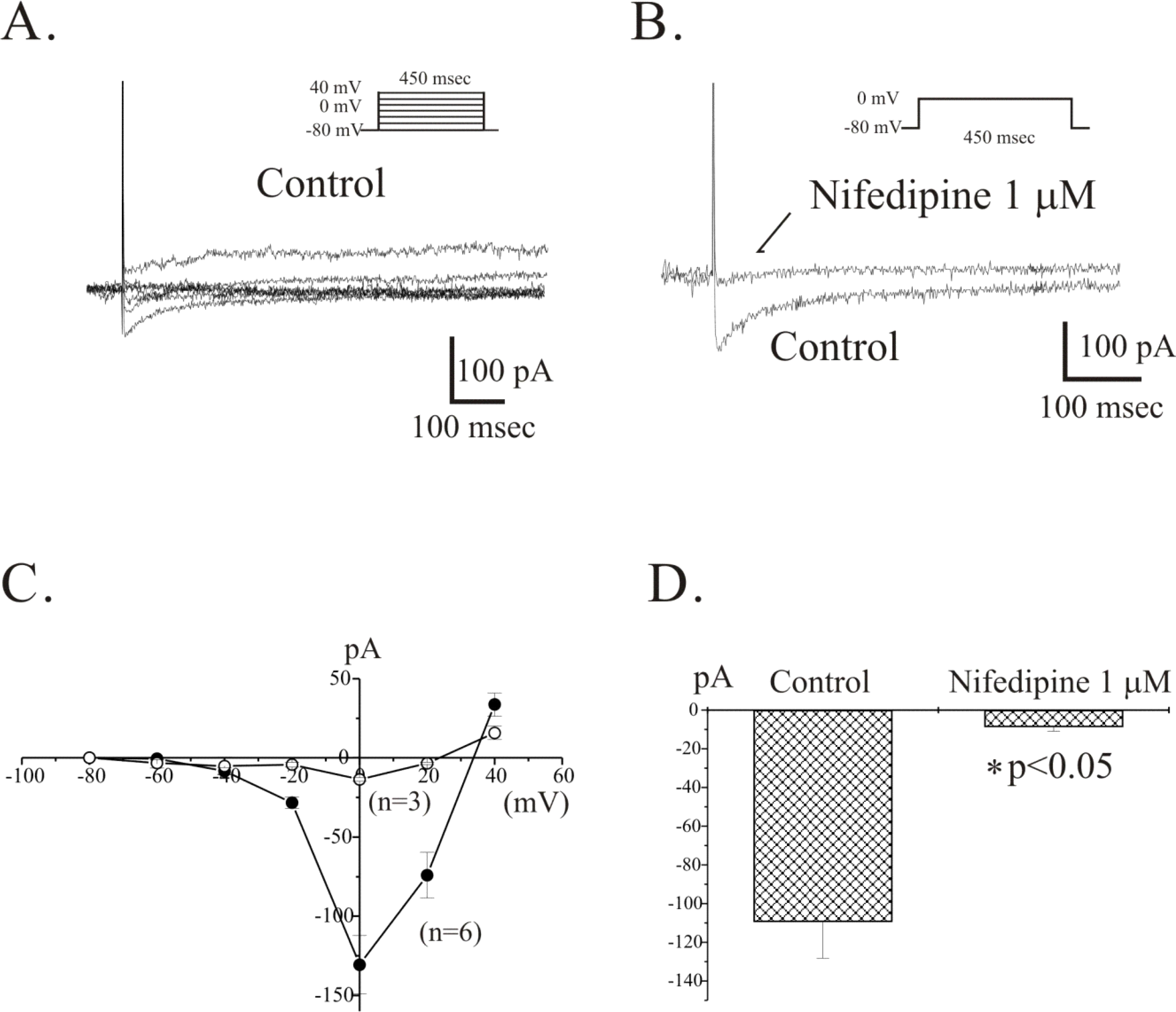
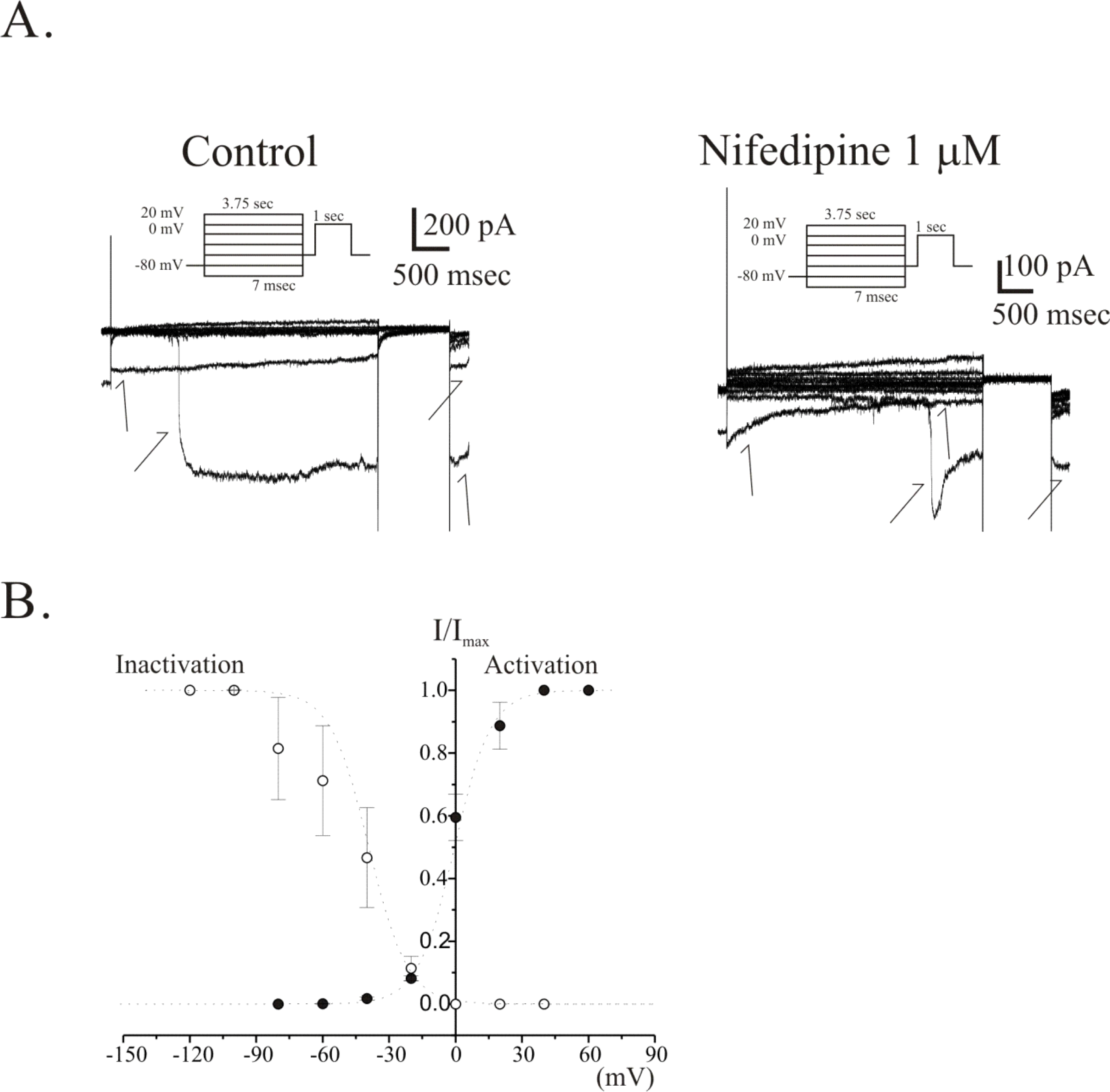
Fig. 6.
Voltage-dependent activation and inactivation kinetics of VDIC in freshly isolated single ICC from guinea-pig antrum. Voltage dependences of activation and inactivation curves of VDIC are shown as a plot of normalized peak currents. (A) Raw current traces recorded for the analysis of steady-state inactivation before (left pannel) and after (right pannel) treatment of 1 μM nifedipine are shown. Note that generation of spontaneous inward current during the recording of VDCC (see arrows in (A)). (B) The steady-state activation and inactivation curves in the absence of nifedipine are plotted and displayed. Filled circles and open circles represent the activation and the inactivation curve, respectively. In the absence of nifedipine, a half-activation and -inactivation voltage (V0.5) were −1±2.9 mV and −48±11 mV, respectively. And slope factors (k) of them were 8±1.6 and 9±1.2, respectively.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download