Abstract
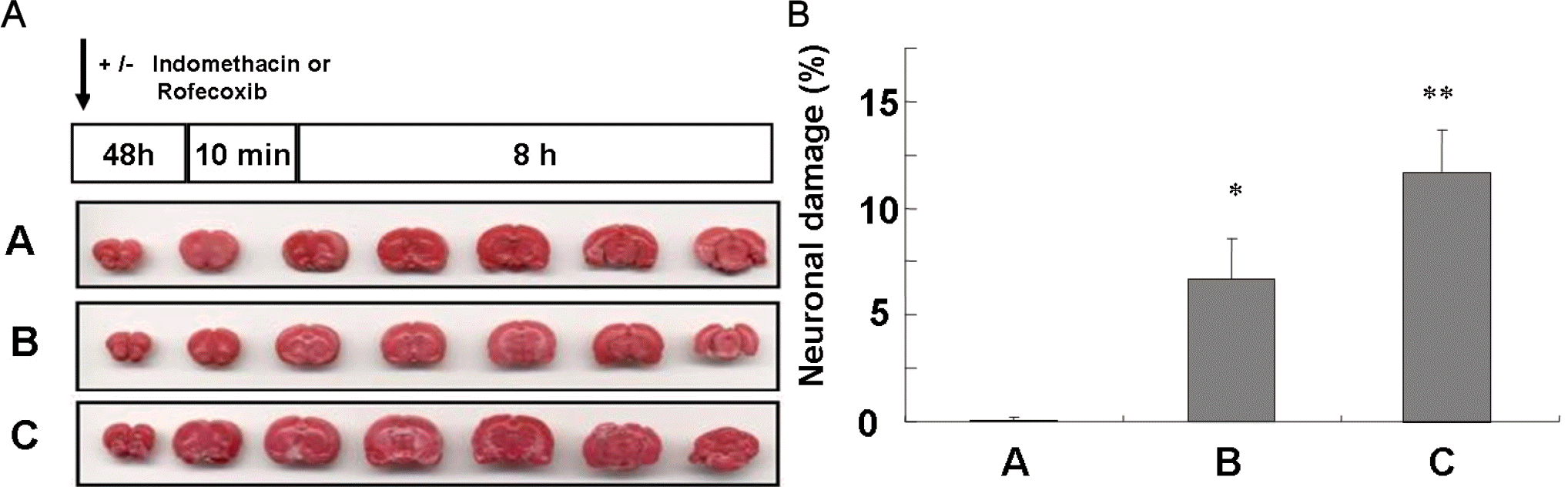
A brief ischemic insult induces significant protection against subsequent massive ischemic events. The molecular mechanisms known as preconditioning (PC)-induced ischemic tolerance are not completely understood. We investigated whether kinetic changes of cyclooxygenase (COX)-2 during reperfusion time-periods after PC were related to ischemic tolerance. Rats were given PC by occlusion of middle cerebral artery (MCAO) for 10 min and sacrificed after the indicated time-periods of reperfusion (1, 2, 4, 8, 12, 18 or 24 h). In PC-treated rats, focal ischemia was induced by occlusion of MCA for 24 h and brain infarct volume was then studied to determine whether different reperfusion time influenced the damage. We report that the most significant protection against focal ischemia was obtained in rats with 8 h reperfusion after PC. Administration of indomethacin (10 mg/kg, oral) or rofecoxib (5 mg/kg, oral) 48 h prior to PC counteracted the effect of PC. Immunohistochemical analysis showed that COX-2 and HO-1 protein were induced in PC-treated rat brain, which was significantly inhibited by rofecoxib. Taken together, we concluded that the kinetic changes of COX-2 expression during the reperfusion period after PC might be partly responsible for ischemic tolerance.
REFERENCES
Barone FC., Feuestein GZ. Inflammatory mediators and stroke: new opportunities for novel therapeutics. J Cereb Blood Flow Metab. 19:819–834. 1999.

Bernaudin M., Tang Y., Reilly M., Petit E., Sharp FR. Brain genomic response following hypoxia and re-oxygenation in the neonatal rat. Identification of genes that might contribute to hypoxia-induced ischemic tolerance. J Biol Chem. 277:39728–39738. 2002.
Choi HC., Kim HS., Lee KY., Chang KC., Kang YJ. NS-398 inhibits proliferation of vascular smooth muscle cells in response to IL-1-by induction of HO-1β. Biochem Biophys Res Commun. 376:753–757. 2008.
Danton GH., Dietrich WD. Inflammatory mechanisms after ischemia and stroke. J Neuropathol Exp Neurol. 62:127–136. 2003.

Dhodda VK., Sailor KA., Bowen KK., Vemuganti R. Putative endogenous mediators of preconditioning-induced ischemic tolerance in rat brain identified by genomic and proteomic analysis. J Neurochem. 89:73–89. 2004.

Edwards RJ., Saurin AT., Rakhit RD., Marber MS. Therapeutic potential of ischemic preconditioning. Br J Clin Pharamcol. 50:87–97. 2000.
Garau A., Bertini R., Colotta F., Casilli F., Bigini P., Cagnotto A., Mennini T., Ghezzi P., Villa P. Neuroprotection with the CXCL8 inhibitor repertaxin in transient brain ischemia. Cytokine. 30:125–131. 2005.

Hong SJ., Li H., Becker KG., Dawson VL., Dawson TM. Identification and analysis of plasticity-induced late-response genes. Proc Natl Acad Sci USA. 101:2145–2150. 2004.

Khan M., Sekhon B., Giri S., Jatana M., Gilg AG., Ayasolla K., Elango C., Singh AK., Singh I. S-Nitrosoglutathione reduces inflammation and protects brain against focal cerebral ischemia in a rat model of experimental stroke. J Cereb Blood Flow Metab. 25:177–192. 2005.

Koistinaho J., Koponen S., Chan PH. Expression of cyclooxygenase-2 mRNA after global ischemia is regulated by AMPA receptors and glucocorticoids. Stroke. 30:1900–1905. 1999.

Monje ML., Toda H., Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 302:1760–1765. 2003.

Guide for the care and Use of Laboratory Animals. NRC [National Research Council]. 7th ed.National Academy Press;Washington DC: 1996.
Planas AM., Soriano MA., Justicia C., Rodríguez-Farré E. Induction of cyclooxygenase-2 in the rat brain after a mild episode of focal ischemia without tissue inflammation or neural cell damage. Neurosci Lett. 275:141–144. 1999.

Ray WA., Stein CM., Daugherty JR., Hall K., Arbogast PG., Griffin MR. COX-2 selective non-steroidal anti-inflammatory drugs and risk of serious coronary heart disease. Lancet. 360:1071–1073. 2002.

Shinmura K., Tang XL., Wang Y., Xuan YT., Liu SQ., Takano H., Bhatnagar A., Bolli R. Cyclooxygenase-2 mediates the cardio-protective effects of the late phase of ischemic preconditioning in conscious rabbits. Proc Natl Acad Sci U S A. 97:10197–10202. 2000.

Truettner J., Busto R., Zhao W., Ginsberg MD., Pérez-Pinzón MA. Effect of ischemic preconditioning on the expression of putative neuroprotective genes in the rat brain. Brain Res Mol Brain Res. 103:106–115. 2002.

Veldhuis WB., Derksen JW., Floris S., Van Der Meide PH., De Vries., J Schepers HE., Vos IM., Dijkstra CD., Kappelle LJ., Nicolay K., Bar PR. Interferon-beta blocks infiltration of inflammatory cells and reduces infarct volume after ischemic stroke in the rat. J Cereb Blood Flow Metab. 23:1029–1039. 2003.

Xu Z., Ford GD., Croslan DR., Jiang J., Gates A., Allen R., Ford BD. Neuroprotection by neuregulin-1 following focal stroke is associated with the attenuation of ischemia-induced pro-inflammatory and stress gene expression. Neurobiol Dis. 19:461–470. 2005.

Yenari MA., Liu J., Zheng Z., Vexler ZS., Lee JE., Giffard RG. Antiapoptotic and anti-inflammatory mechanisms of heat-shock protein protection. Ann NY Acad Sci. 1053:74–83. 2005.

Yrjanheikki J., Tikka T., Keinanen R., Goldsteins G., Chan PH., Koistinaho J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci USA. 96:13496–13500. 1999.

Zhang W., Stanimirovic D. Current and future therapeutic strategies to target inflammation in stroke. Curr Drug Targets Inflamm Allergy. 1:151–166. 2002.
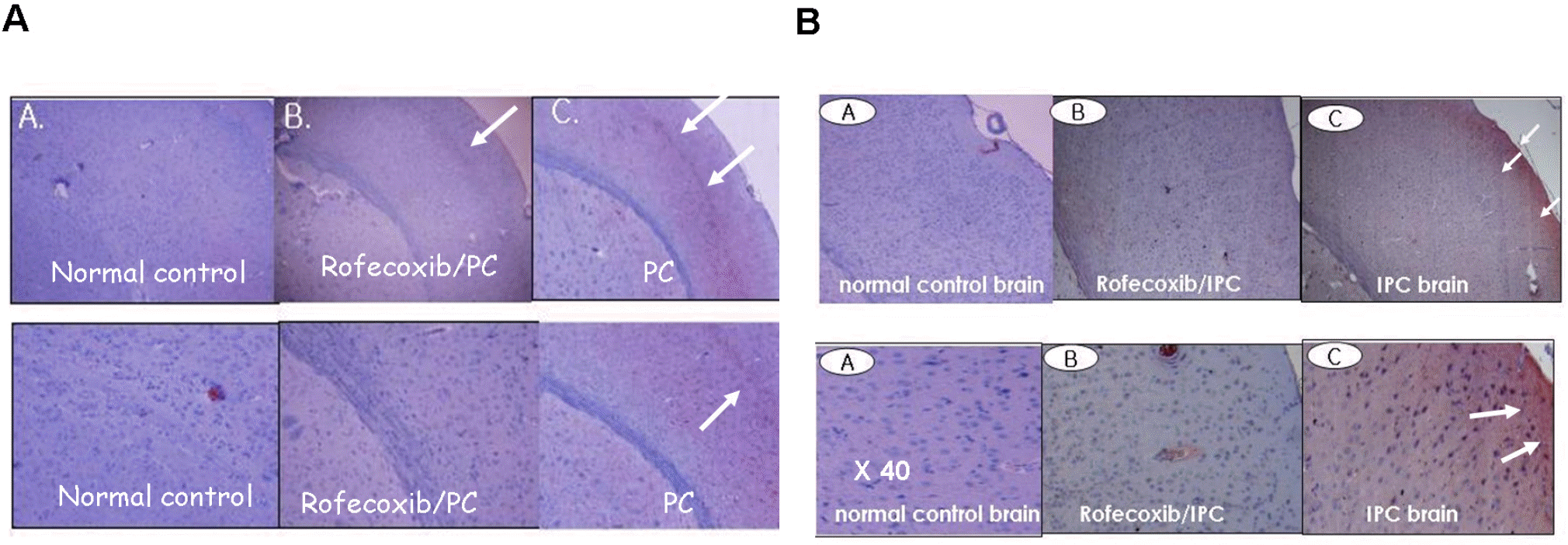
Fig. 1.
Time course of COX-2 expression by Western blot analysis in rat brain subjected to MCA occlusion for 10 min. The results were presented as ratio of COX-2/β-actin, in which the amount of constitutively expressed COX-2/β-actin at 0 h was designated as 1. It should be noted that preconditioning increases COX-2 expression which varied depending on the reperfusion.
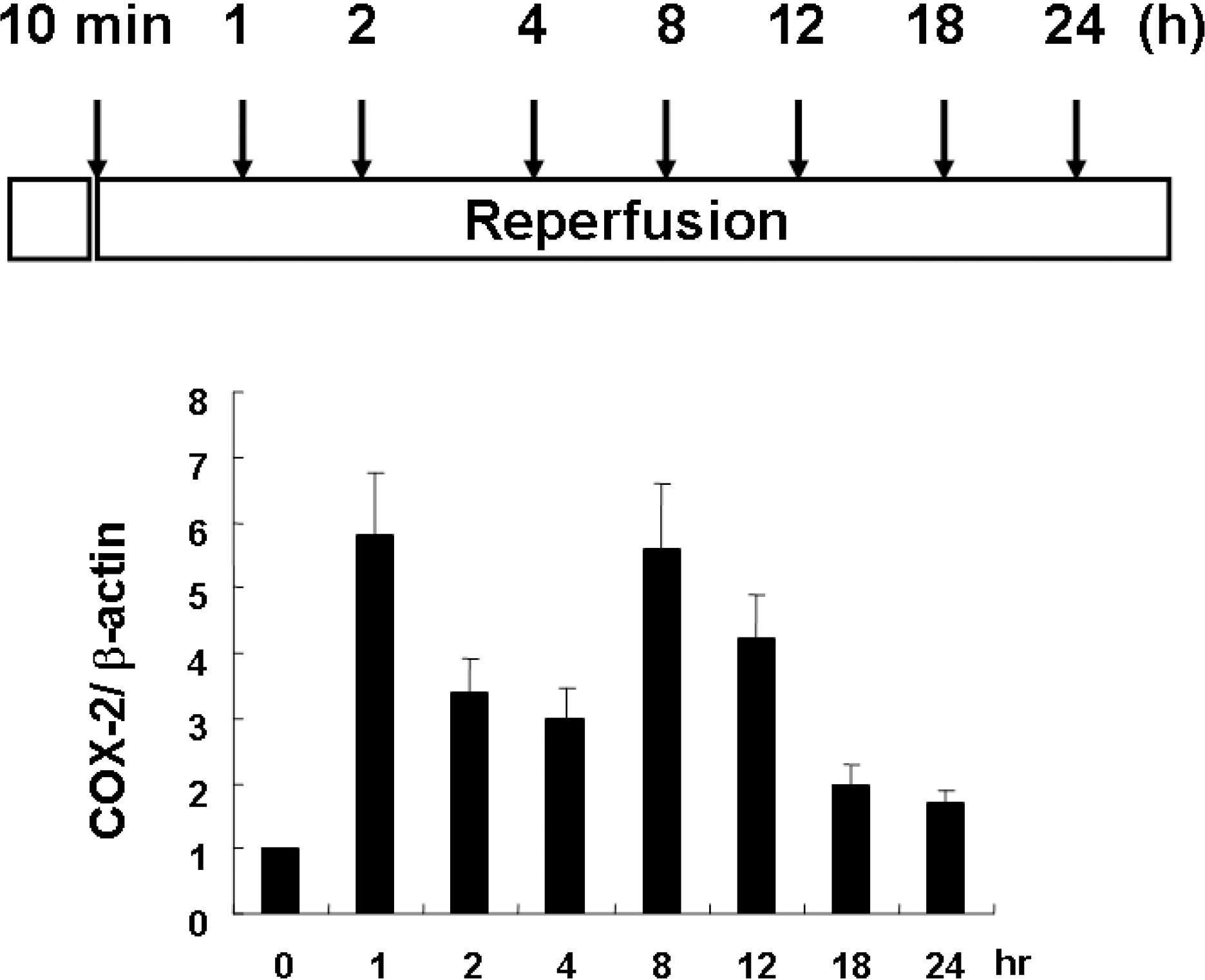
Fig. 2.
A representative photograph of neuronal damage between sham-operated (A) and ischemic preconditioned rat brain (B), in which 8 h reperfusion was performed after 10 min MCA occlusion.
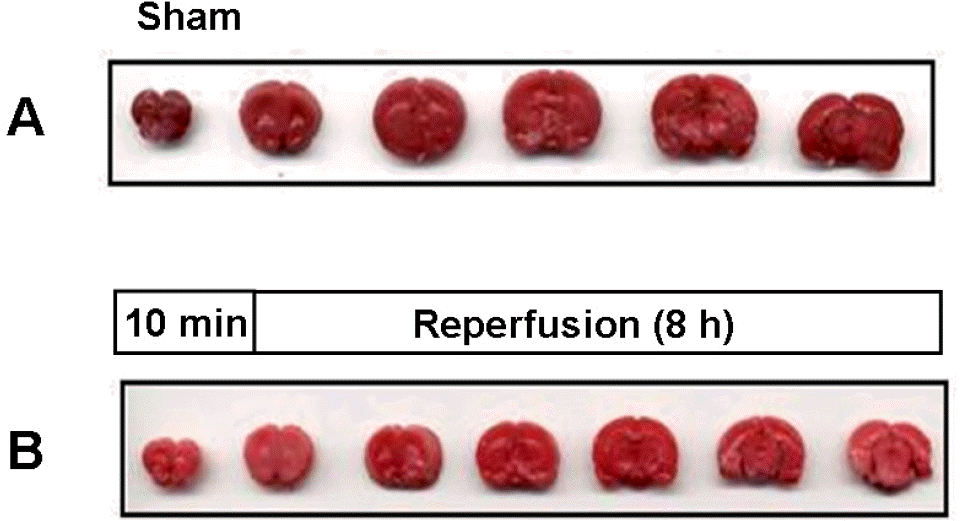
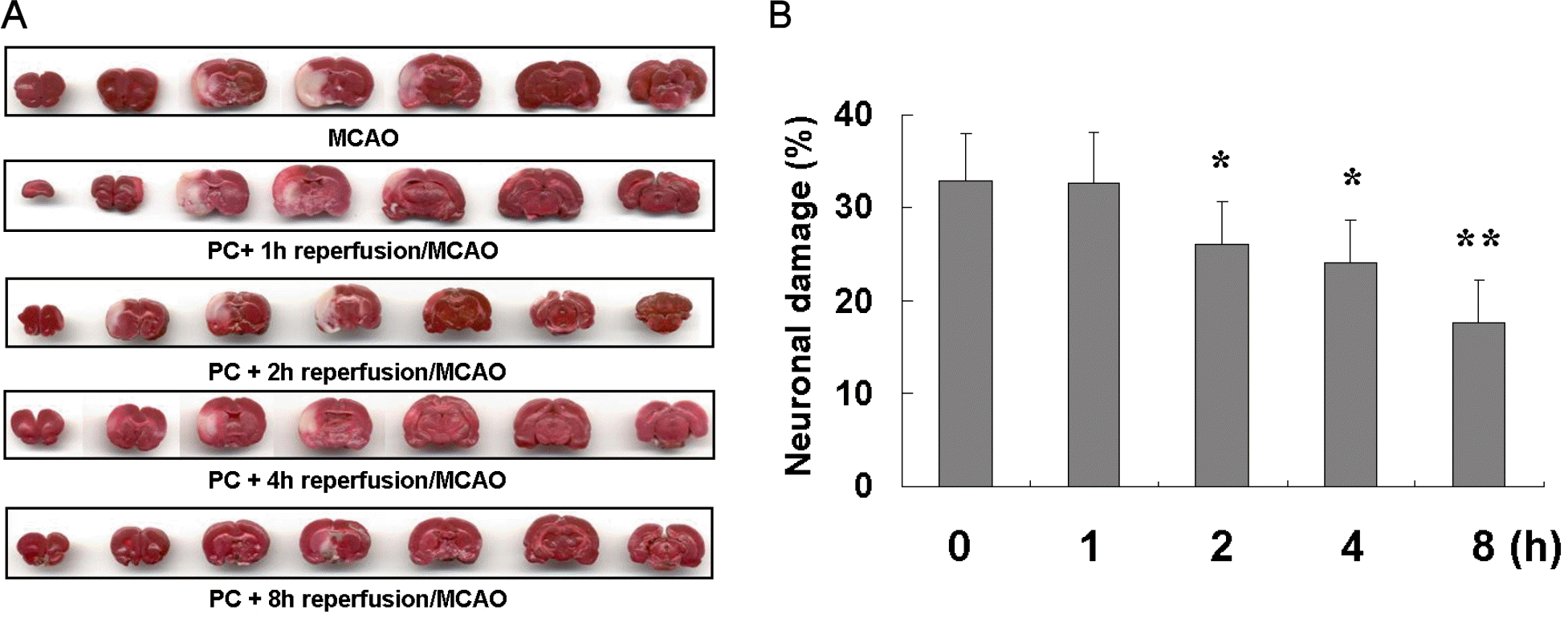
Fig. 3.
Size of brain infarction according to reperfusion time after PC against focal ischemia (24 h, MCAO). (A). Percentile of neuronal damage (%) occurred with different time of reperfusion after ischemic preconditioning against focal ischemia (B).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download