Abstract
Since first discovered in chick skeletal muscles, stretch-activated channels (SACs) have been proposed as a probable mechano-transducer of the mechanical stimulus at the cellular level. Channel properties have been studied in both the single-channel and the whole-cell level. There is growing evidence to indicate that major stretch-induced changes in electrical activity are mediated by activation of these channels. We aimed to investigate the mechanism of stretch-induced automaticity by exploiting a recent mathematical model of rat atrial myocytes which had been established to reproduce cellular activities such as the action potential, Ca2+ transients, and contractile force. The incorporation of SACs into the mathematical model, based on experimental results, successfully reproduced the repetitive firing of spontaneous action potentials by stretch. The induced automaticity was composed of two phases. The early phase was driven by increased background conductance of voltage-gated Na+ channel, whereas the later phase was driven by the reverse-mode operation of Na+/Ca2+ exchange current secondary to the accumulation of Na+ and Ca2+ through SACs. These results of simulation successfully demonstrate how the SACs can induce automaticity in a single atrial myocyte which may act as a focus to initiate and maintain atrial fibrillation in concert with other arrhythmogenic changes in the heart.
REFERENCES
Baruscotti M., DiFrancesco D., Robinson RB. Na+ current contribution to the diastolic depolarization in newborn rabbit SA node cells. Am J Physiol. 279:H2303–H2309. 2000.
Baumgarten CM., Clemo HF. Swelling-activated chloride channels in cardiac physiology and pathophysiology. Prog Biophys Mol Biol. 82:25–42. 2003.

Bode F., Katchman A., Woosley RL., Franz MR. Gadolinium decreases stretch-induced vulnerability to atrial fibrillation. Circulation. 101:2200–2205. 2000.

Bode F., Sachs F., Franz MR. Tarantula peptide inhibits atrial fibrillation. Nature. 409:35–36. 2001.

Boland J., Troquet J. Intracellular action potential changes induced in both ventricles of the rat by an acute right ventricular pressure overload. Cardiovasc Res. 14:735–740. 1980.

Boyle WA., Nerbonne JM. Two functionally distinct 4-aminopyridine-sensitive outward K+ currents in rat atrial myocytes. J Gen Physiol. 100:1041–1067. 1992.
Bustamante JO., Ruknudin A., Sachs F. Stretch-activated channels in heart cells: relevance to cardiac hypertrophy. J Cardiovasc Pharmacol. 17:S110–S113. 1991.
Dean JW., Lab MJ. Arrhythmia in heart failure: role of mechanically induced changes in electrophysiology. Lancet. 1:1309–1312. 1989.

Ferrier GR. Digitalis arrhythmias: role of oscillatory afterpotentials. Prog Cardiovasc Dis. 19:459–474. 1977.

Franz MR., Bode F. Mechano-electrical feedback underlying arrhythmias: the atrial fibrillation case. Prog Biophys Mol Biol. 82:163–174. 2003.

Franz MR., Burkhoff D., Yue DT., Sagawa K. Mechanically induced action potential changes and arrhythmia in isolated and in situ canine hearts. Cardiovasc Res. 23:213–223. 1989.

Franz MR., Cima R., Wang D., Profitt D., Kurz R. Electrophysiological effects of myocardial stretch and mechanical determinants of stretch-activated arrhythmias [published erratum appears in Circulation 1992; 86:1663]. Circulation. 86:968–978. 1992.
Hansen DE. Mechanoelectrical feedback effects of altering preload, afterload, and ventricular shortening. Am J Physiol. 264:H423–H432. 1993.

Hordof AJ., Spotnitz A., Mary-Rabine L., Edie RN., Rosen MR. The cellular electrophysiologic effects of digitalis on human atrial fibers. Circulation. 57:223–229. 1978.

Hove-Madsen L., Llach A., Bayes-Genís A., Roura S., Rodriguez Font E., Arís A., Cinca J. Atrial fibrillation is associated with increased spontaneous calcium release from the sarcoplasmic reticulum in human atrial myocytes. Circulation. 110:1358–1363. 2004.

Hoyer J., Distler A., Haase W., Gögelein H. Ca2+ influx through stretch-activated cation channels activates maxi K+ channels in porcine endocardial endothelium. Proc Natl Acad Sci USA. 91:2367–2371.
Hu H., Sachs F. Mechanically activated currents in chick heart cells. J Membr Biol. 154:205–216. 1996.

Isenberg G., Kazanski V., Kondratev D., Gallitelli MF., Kiseleva I., Kamkin A. Differential effects of stretch and compression on membrane currents and [Na+]c in ventricular myocytes. Prog Biophys Mol Biol. 82:43–56. 2003.
Kaufmann R., Theophile U. Automatiefördernde Dehnungseffekte an Purkinje-Fäden, Papillarmuskeln und Vorhoftrabekeln von Rhesus-Affen. Pflügers Arch. 297:174–189. 1967.

Kiseleva I., Kamkin A., Wagner KD., Theres H., Ladhoff A., Scholz H., Günther J., Lab MJ. Mechanoelectric feedback after left ventricular infarction in rats. Cardiovasc Res. 45:370–378. 2000.

Lab MJ. Mechanically dependent changes in action potentials recorded from the intact frog ventricle. Circ Res. 42:519–528. 1978.

Lei M., Jones SA., Liu J., Lancaster MK., Fung SS., Dobrzynski H., Camelliti P., Maier SK., Noble D., Boyett MR. Requirement of neuronal- and cardiac-type sodium channels for murine sinoatrial node pacemaking. J Physiol. 559:835–848. 2004.

Maier SK., Westenbroek RE., Yamanushi TT., Dobrzynski H., Boyett MR., Catterall WA., Scheuer T. An unexpected requirement for brain-type sodium channels for control of heart rate in the mouse sinoatrial node. Proc Natl Acad Sci USA. 100:3507–3512. 2003.

Maltsev VA., Lakatta EG. Dynamic interactions of an intracellular Ca2+ clock and membrane ion channel clock underlie robust initiation and regulation of cardiac pacemaker function. Cardiovasc Res. 77:274–284. 2008.
Mary-Rabine L., Albert A., Pham TD., Hordof A., Fenoglio JJ Jr., Malm JR., Rosen MR. The relationship of human atrial cellular electrophysiology to clinical function and ultrastructure. Circ Res. 52:188–199. 1983.

Matsuoka S., Sarai N., Kuratomi S., Ono K., Noma A. Role of individual ionic current systems in ventricular cells hypothesized by a model study. Jpn J Physiol. 53:105–123. 2003.

Niu W., Sachs F. Dynamic properties of stretch-activated K+ channels in adult rat atrial myocytes. Prog Biophys Mol Biol. 82:121–135. 2003.
Noble D. Simulation of Na/Ca exchange activity during ischemia. Ann N Y Acad Sci. 976:431–437. 2002.

Noble D., Noble PJ. Late sodium current in the pathophysiology of cardiovascular disease: consequences of sodium-calcium overload. Heart. 92:iv1–iv5. 2006.

Nuss HB., Balser JR., Orias DW., Lawrence JH., Tomaselli GF., Marban E. Coupling between fast and slow inactivation revealed by analysis of a point mutation (F1304Q) in μl rat skeletal muscle sodium channels. J Physiol. 494:411–429. 1996.
Prakash P., Meera P., Tripathi O. Effects of calcium channel blockers on spontaneous electrical activity of freshly isolated three-day-old embryonic chick ventricle. Reprod Fertil Dev. 8:921–929. 1996.

Psaty BM., Manolio TA., Kuller LH., Kronmal RA., Cushman M., Fried LP., White R., Furberg CD., Rautaharju PM. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 96:2455–2461. 1997.

Rota M., Vassalle M. Patch-clamp analysis in canine cardiac Purkinje cells of a novel sodium component in the pacemaker range. J Physiol. 548:147–165. 2003.

Sadoshima J., Izumo S. Mechanical stretch rapidly activates multiple signal transduction pathways in cardiac myocytes: potential involvement of an autocrine/paracrine mechanism. EMBO J. 12:1681–1692. 1993.

Sanders L., Rakovic S., Lowe M., Mattick PA., Terrar DA. Fundamental importance of Na+-Ca2+ exchange for the pacemaking mechanism in guinea-pig sino-atrial node. J Physiol. 571:639–649. 2006.
Sarai N., Matsuoka S., Kuratomi S., Ono K., Noma A. Role of individual ionic current systems in the SA node hypothesized by a model study. Jpn J Physiol. 53:125–134. 2003.

Satoh H., Mukai M., Urushida T., Katoh H., Terada H., Hayashi H. Importance of Ca2+ influx by Na+/Ca2+ exchange under normal and sodium-loaded conditions in mammalian ventricles. Mol Cell Biochem. 242:11–17. 2003.
Sorota S. Swelling-induced chloride-sensitive current in canine atrial cells revealed by whole-cell patch-clamp method. Circ Res. 70:679–687. 1992.

Terrenoire C., Lauritzen I., Lesage F., Romey G., Lazdunski M. A TREK-1-like potassium channel in atrial cells inhibited by beta-adrenergic stimulation and activated by volatile anesthetics. Circ Res. 89:336–342. 2001.
Tseng GN. Cell swelling increases membrane conductance of canine cardiac cells: evidence for a volume-sensitive Cl channel. Am J Physiol. 262:C1056–C1068. 1992.

Vandenburgh HH. Mechanical forces and their second messengers in stimulating cell growth in vitro. Am J Physiol. 262:R350–R355. 1992.

Vassalle M., Scidá EE. The role of sodium in spontaneous discharge in the absence and in the presence of strophanthidin. Fed Proc. 38:880. 1979.
Vaziri SM., Larson MG., Benjamin EJ., Levy D. Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study. Circulation. 89:724–730. 1994.

Vinogradova TM., Maltsev VA., Bogdanov KY., Lyashkov AE., Lakatta EG. Rhythmic Ca2+ oscillations drive sinoatrial nodal cell pacemaker function to make the heart tick. Ann N Y Acad Sci. 1047:138–156. 2005.
Wagner MB., Kumar R., Joyner RW., Wang Y. Induced automaticity in isolated rat atrial cells by incorporation of a stretch-activated conductance. Pflügers Arch. 447:819–829. 2004.

Youm JB. Stretch-activated K+ channels in rat atrial myocytes. Korean J Physiol Pharmacol. 7:341–348. 2003.
Fig. 1.
Simulation of SACs-induced automaticity in the heart. Increasing SACs conductance (13.6 μS/μF) triggered repetitive firing of action potentials (APs) in otherwise quiescent model cell of rat atrial myocytes. The AP train is composed of early and delayed phases with different frequency (2.8 Hz vs 1.2 Hz). Upon releasing the SACs activation, the model cell stopped firing, leaving very small depolarizations (A). Contractile force (B) and Ca2+ (C) show time course similar to that of membrane potential during the SACs activation. After release of SACs activation, however, the contractile force and Ca2+ continue oscillation with decreasing frequency. Na+ (D) is slowly accumulated with activation of SACs and decreased slowly on release of activation.
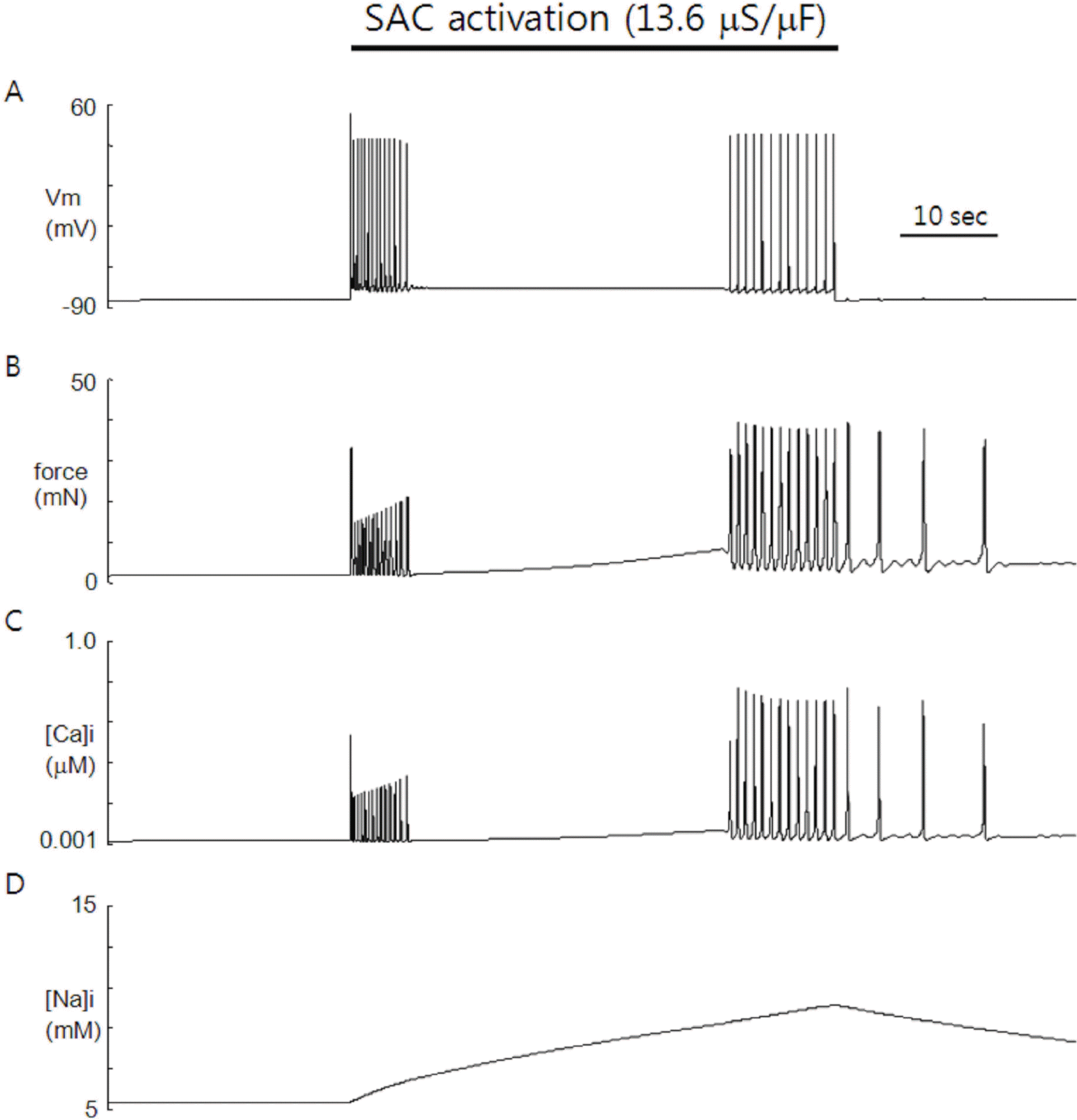
Fig. 2.
Role of INa in the SACs-induced automaticity. The length of early phase in the repetitive firing of APs is dependent on the maximal conductance of voltage-gated Na+ channel. Reducing the maximal conductance to 40% of control failed to generate the repetitive firing of APs. As the maximal conductance of voltage-gated Na+ channel was more increased, however, the automaticity appeared and the length of early phase in repetitive firing of APs was increased. The length of delayed phase was relatively unaffected when the maximal conductance was varied between 80% and 120% relative to the control. When the maximal conductance was increased to 140% relative to the control, two phases merged together.
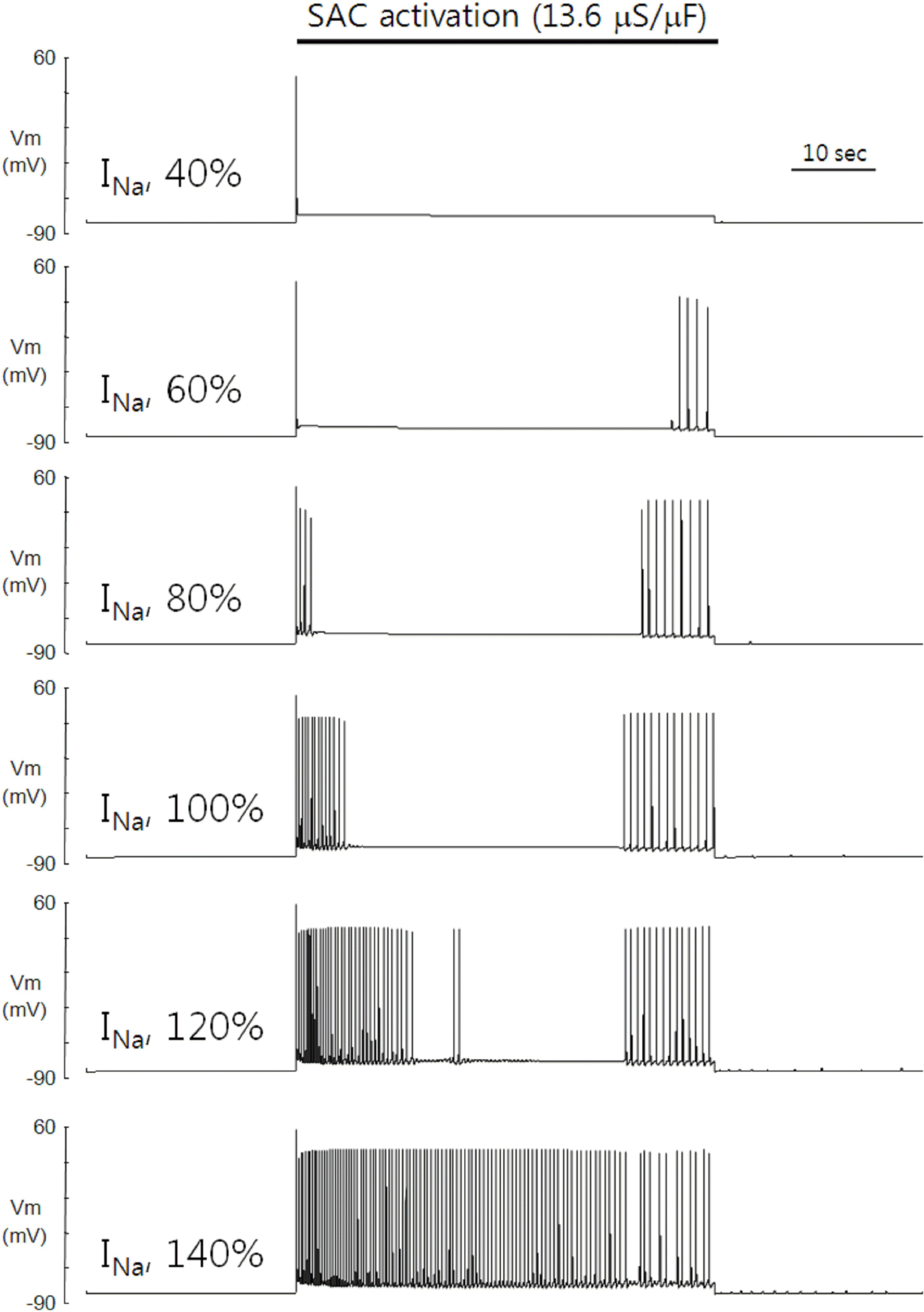
Fig. 3.
Role of INaCa in SACs-induced automaticity. Clamping of INaCa just before the delayed phase of repetitive firing of APs by SACs activation abolished the delayed phase, leaving oscillations of contractile force and Ca2+ unaffected. Time course of [Na+]i was also unaffected compared with that of the control (see Fig. 1D).
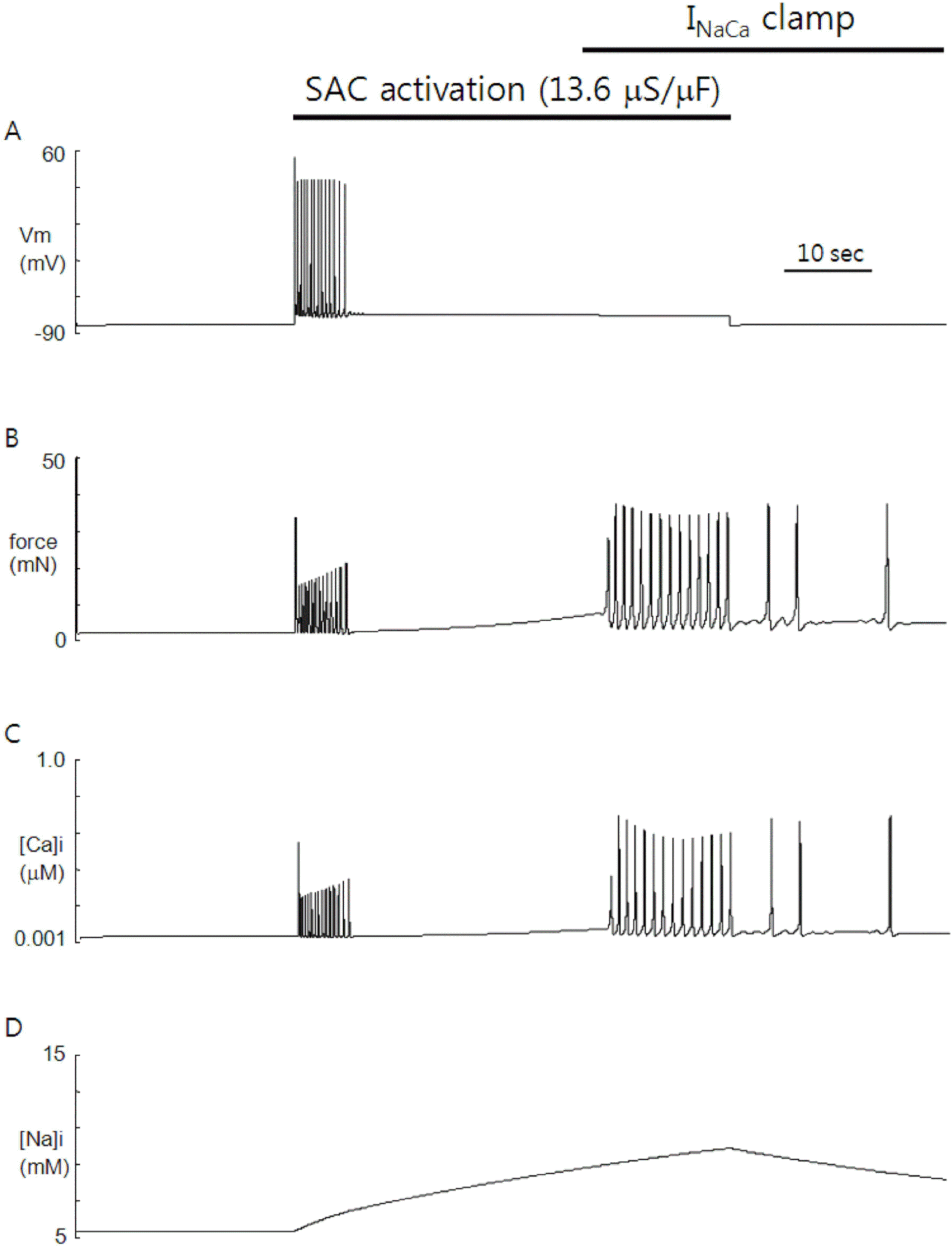
Fig. 4.
Role of intracellular cations in the SACs-induced automaticity. Clamping of cation concentrations during the SACs activation abolished the delayed phase of SACs-induced automaticity, leaving only the early phase under conditions of 60% (A) and 80% (B) in maximal conductance of voltage-gated Na+ channel relative to the control. The firing of APs continued during the SACs activation under the condition of 100% (C) in maximal conductance relative to the control, indicating that the early phase is driven by purely electrical means.
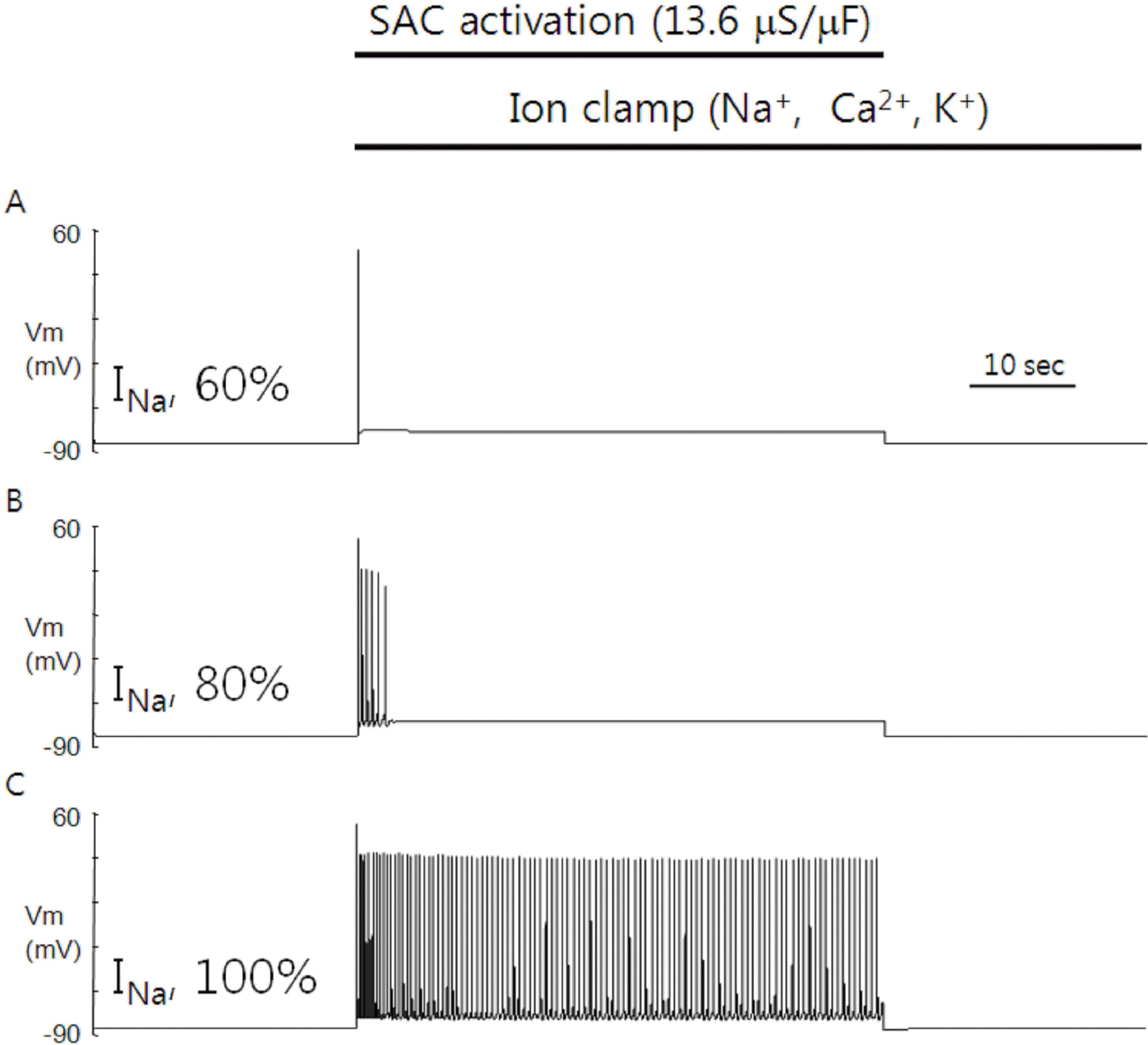
Table 1.
Parameter details
Table 2.
Definition of symbols




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download