Abstract
Although many studies show that thromboxane A2 (TXA2) has the action of gastrointestinal (GI) motility using GI muscle cells and tissue, there are no reports on the effects of TXA2 on interstitial cells of Cajal (ICC) that function as pacemaker cells in GI tract. So, we studied the modulation of pacemaker activities by TXA2 in ICC with whole cell patch-clamp technique. Externally applied TXA2 (5μM) produced membrane depolarization in current-clamp mode and increased tonic inward pacemaker currents in voltage-clamp mode. The tonic inward currents by TXA2 were inhibited by intracellular application of GDP-β-S. The pretreatment of ICC with Ca2+ free solution and thapsigargin, a Ca2+-ATPase inhibitor in endoplasmic reticulum, abolished the generation of pacemaker currents and suppressed the TXA2-induced tonic inward currents. However, chelerythrine or calphostin C, protein kinase C inhibitors, did not block the TXA2-induced effects on pacemaker currents. These results suggest that TXA2 can regulate intestinal motility through the modulation of ICC pacemaker activities. This modulation of pacemaker activities by TXA2 may occur by the activation of G protein and PKC independent pathway via extra and intracellular Ca2+ modulation.
REFERENCES
Arita H., Nakano T., Hanasaki K. Thromboxane A2: its generation and role in platelet activation. Prog Lipid Res. 28:273–301. 1989.
Bennett A., Elev KG., Scholes GB. Effects of prostaglandins E1 and E2 on human, guinea pig and rat isolated small intestine. Br J Pharmacol. 34:630–638. 1968.
Bennett A., Hensy CN., Sanger GJ., Stamford IF. Metabolites of arachidonic acid formed by human gastrointestinal tissues and their actions on the muscle layers. Br J Pharmacol. 74:435–444. 1981.

Bennett A., Sanger GJ. Pinane thromboxane A2 analogues are non-selective prostanoid antagonists in rat and human stomach muscle. Br J Pharmacol. 77:591–596. 1982.
Berezin I., Daniel EE., Huizinga JD. Ultrastructure of interstitial cells of Cajal in the canine distal esophagus. Can J Physiol Pharmacol. 72:1049–1059. 1994.

Berezin I., Huizinga JD., Daniel EE. Interstitial cells of Cajal in the canine colon: a special communication network at the inner border of the circular muscle. J Comp Neurol. 273:42–51. 1988.

Brass LF., Shaller CC., Belmonte EJ. Inositol 1,4,5-triphosphate induced granule secretion in platelets, Evidende that the activation of phospholipase C mediated by platelet thromboxane receptors involves a guanine nucleotide binding protein-dependent mechanism distinct from that of thrombin. J Clin Invest. 79:1269–1275. 1987.
Coleman RA., Humphrey PP., Kennedy I., Levy GP., Lumley P. Comparison of the actions of U-46619, a prostaglandin H2-analogue, with those of prostaglandin H2 and thromboxane A2 on some isolated smooth muscle preparations. Br J Pharmacol. 73:773–778. 1981.
Daniel EE., Posey-Daniel V. Neuromuscular structures in opossum esophagus: role of interstitial cells of Cajal. Am J Physiol. 246:G305–G315. 1984.

Ferreira SH., Herman AG., Vane JR. Prostaglandin production by rabbit isolated jejunum and its relationship to the inherent tone of the preparation. Br J Pharmacol. 56:469–477. 1976.

Koh SD., Sanders KM., Ward SM. Spontaneous electrical rhythmicity in cultured interstitial cells of Cajal from the mouse small intestine. J Physiol. 513:203–213. 1998.
LeDuc LE., Needleman P. Regional localization of prostacyclin and thromboxane synthesis in dog stomach and intestinal tract. J Pharmacol Exp Ther. 211:181–188. 1979.
Nakano T., Hanasaki K., Arita H. Different effects of two thromboxane A2/prostaglandin H2 receptor ligand, U46619 and S-14, on rabbit platelets. FEBS Lett. 234:309–312. 1988.
Needleman P., Turk J., Jakschik BA., Morrison AR., Lefkowith JB. Arachidonic acid metabolism. Annu Rev Biochem. 55:69–109. 1986.

Okada Y., Hara A., Ma H., Xiao CY., Takahata O., Kohgo Y., Narumiya S., Ushikubi F. Characterization of prostanoid receptors mediating contraction of the gastric fundus and ileum: studies using mice deficient in prostanoid receptors. Br J Pharmacol. 131:745–755. 2000.

Pollock WK., Armstrong RA., Brydon LJ., Jones RL., Macintyre DE. Thromboxane-induced phosphatide formation in human platelets. Relationship to receptor occupancy and to changes in cytosolic free calcium. Biochem J. 219:833–842. 1984.
Portbury AL., Furness JB., Young HM., Southwell BR., Vigna SR. Localization of NK1 receptor immunoreactivity to neurons and interstitial cells of the guinea-pig gastrointestinal tract. J Comp Neurol. 367:342–351. 1996.
Publicover NG., Horowitz NN., Sanders KM. Calcium oscillations in freshly dispersed and cultured interstitial cells of from canine colon. Am J Physiol. 262:C589–C597. 1992.
Robert A. Prostaglandins and the gastrointestinal tract. Hohnson LR ed, Physiology of the Gastrointestinal Tract. 1st ed.Raven;New York: p. p. 1407–1434. 1981.
Sage SO., Rink TJ. The kinetics of changes in intracellular calcium concentration in fura-2-loaded human platelets. J Biol Chem. 262:16364–16369. 1987.

Sanders KM. Evidence that endogenous prostacyclin modulates the electrical and mechanical activities of canine ileal circular muscle. J Gastroenterol. 19:401. 1981.
Sanders KM. Evidence that prostaglandins are local regulatory agents in canine ileal circular muscle. Am J Physiol. 246:G361–G371. 1984.

Sanders KM. Ionic mechanisms of electrical rhythmicity in gastrointestinal smooth muscles. Annu Rev Physiol. 54:439–453. 1992.

Shenker A., Goldsmith P., Unson CG., Spiegel AM. The G protein coupled to the thromboxane A2 receptor in human platelets is a member of the novel Gq family. J Biol Chem. 266:9309–9313. 1991.
Shuttleworth CWR., Xue C., Ward SM., de Vente J., Sanders KM. Immunohistochemical localization of 3',5'-cyclic guanosine monophosphate in the canine proximal colon: responses to nitric oxide and electrical stimulation of enteric inhibitory neurons. Neuroscience. 56:513–522. 1993.

Siess W., Stifel M., Binder H., Weber PC. Activation of V1-receptors by vasopressin stimulates inositol phospholipids hydrolysis and arachidonate metabolism in human platelets. Biochem J. 233:83–91. 1986.
Sternini C., Su D., Gamp PD., Bunnett NW. Cellular sites of expression of the neurokinin-1 receptor in the rat gastrointestinal tract. J Comp Neurol. 258:531–540. 1995.

Thomsen L., Robinson TL., Lee JCF., Farraway L., Hughes MJH., Andrews DW., Huizinga JD. Interstitial cells of Cajal generate a rhythmic pacemaker current. Nature Med. 4:448–451. 1998.
Thuneberg L. Interstitial cells of Cajal: intestinal pacemakers? Adv Anat Embryol Cell Biol. 71:11–30. 1982.
Torihashi S., Kobayashi S., Gerthoffer WT., Sanders KM. Interstitial cells in deep muscular plexus of canine small intestine may be specialized smooth muscle cells. Am J Physiol. 265:G638–G645. 1993.

Watson SP., Reep B., McConnell RT., Lapetina EG. Collagen stimulates [3H]inositol trisphosphate formaton in indomethacin-treated human platelets. Biochem J. 226:831–837. 1985.
Fig. 1.
The effects of TXA2 on pacemaker potentials and pacemaker currents recorded in cultured ICC from mouse small intestine (A) Pacemaker potentials of ICC which were exposed to TXA2 (5 μM) in the current-clamping mode (I=0). Vertical solid line scales amplitude of pacemaker potential and horizontal solid line scales for duration of recording (s) pacemaker potentials. (B), (C), and (D) Pacemaker currents of ICC recorded at a holding potential of −70 mV, when exposed to various concentrations of TXA2 (1, 3, and 5 μM). The dotted lines indicate zero current levels. Vertical solid line scales amplitude of pacemaker current and horizontal solid line scales duration of recording (s) pacemaker currents. The responses to TXA2 are summarized in (E), (F) and (G). The bars represent mean values±SE. ∗∗Significantly different from the untreated control (Con) (p < 0.01).
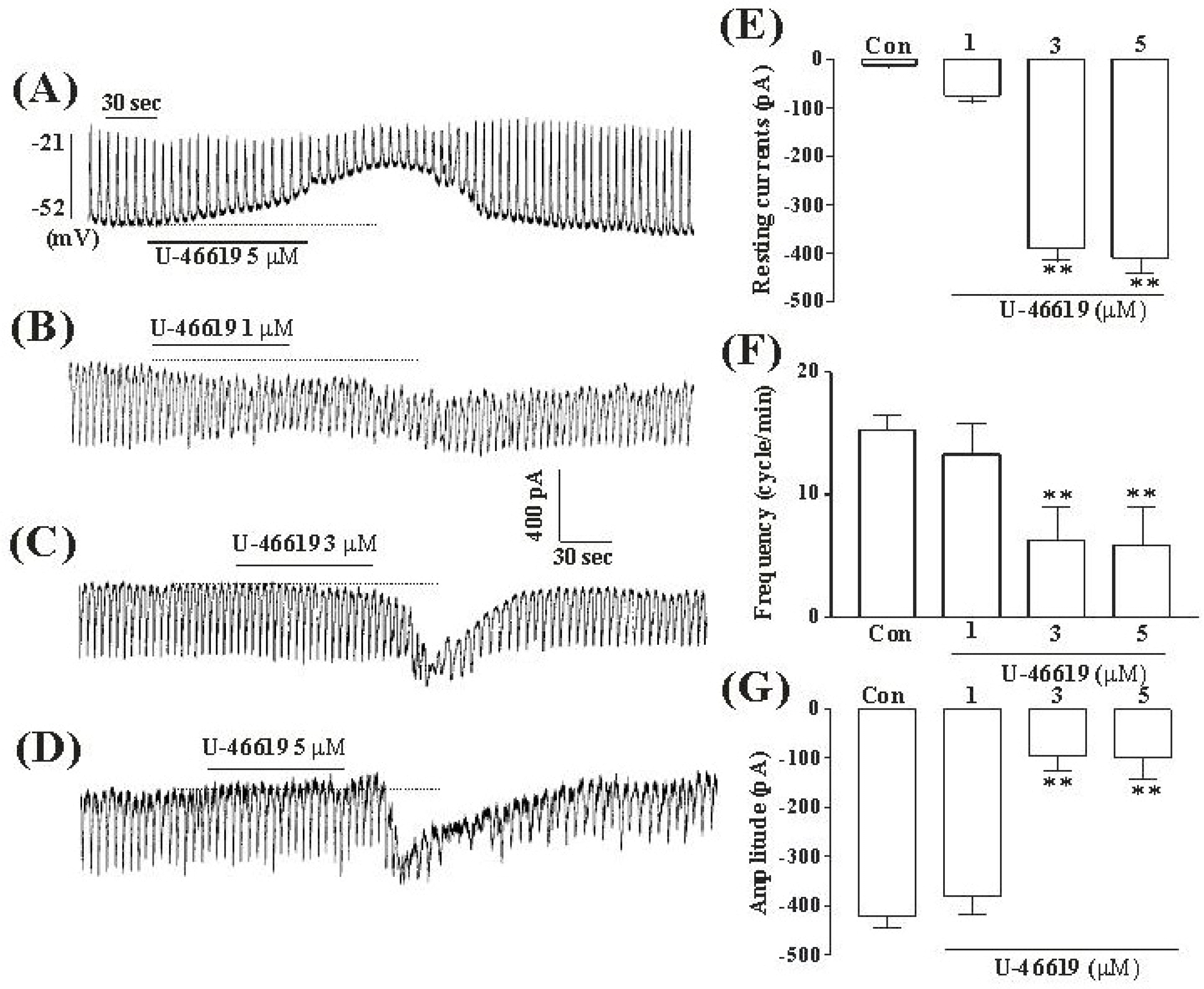
Fig. 2.
The effects of GDP-β-S in response to TXA2 induced pacemaker currents from ICC of mouse small intestine (A) Pacemaker currents from ICC exposed to TXA2 (5 μM) in the presence of GDP-β-S (1 mM) in the pipette. The tonic inward currents with suppressed amplitudes and frequency induced by TXA2 were blocked by internally applied GDP-β-S (1 mM). The dotted lines indicate the zero current levels. The effects of TXA2 in the presence of GDP-β-S are summarized in (B). Vertical solid line scales amplitude of pacemaker current and horizontal solid line scales for duration of recording (s) pacemaker currents. Bars represent mean values ± SE. The effects of GDP-β-S on TXA2-induced pacemaker currents were significantly different from the TXA2-induced pacemaker currents (p < 0.01). The bars represent mean values±SE. ∗∗Significantly different from the untreated control (Con) (p < 0.01).
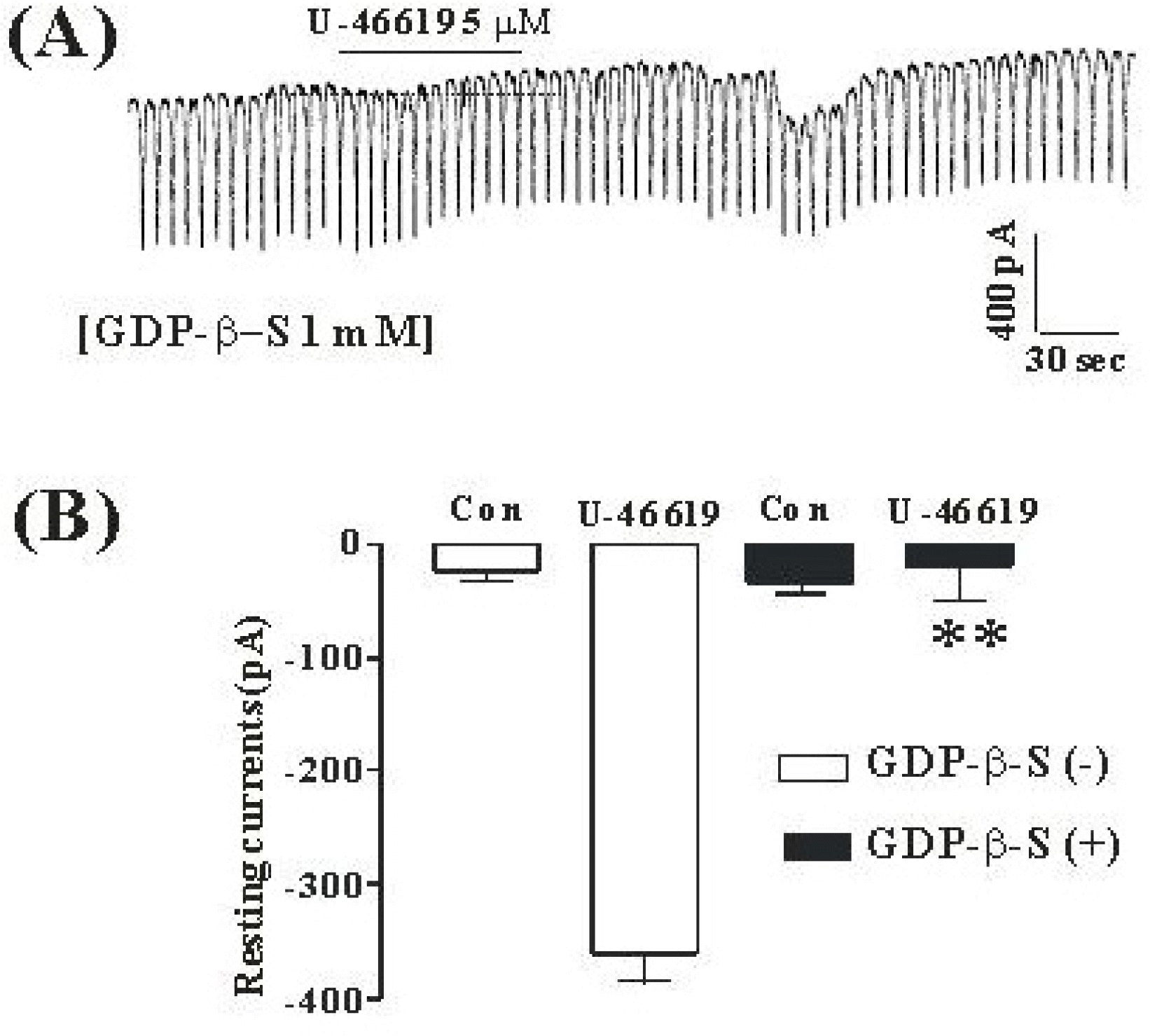
Fig. 3.
The effects of an external Ca2+-free solution or thapsigargin on the TXA2-induced response on pacemaker currents in cultured ICC from mouse small intestine (A) External Ca2+-free solution abolished the generation of pacemaker currents. Under this condition, the TXA2 (5μM)-induced tonic inward currents were blocked. (C) Thapsigargin (5μM) abolished the generation of pacemaker currents. Thapsigargin also blocked the TXA2 (5μM)-induced tonic inward currents. The dotted lines indicate the zero current levels. Responses to the TXA2 in the external Ca2+-free solution and in the presence of thapsigargin are summarized in (B) and (D). Vertical solid line scales amplitude of pacemaker current and horizontal solid line scales duration of recording (s) pacemaker current. The bars represent mean values ± SE. ∗∗Significantly different from the untreated Control (Con) (p < 0.01).
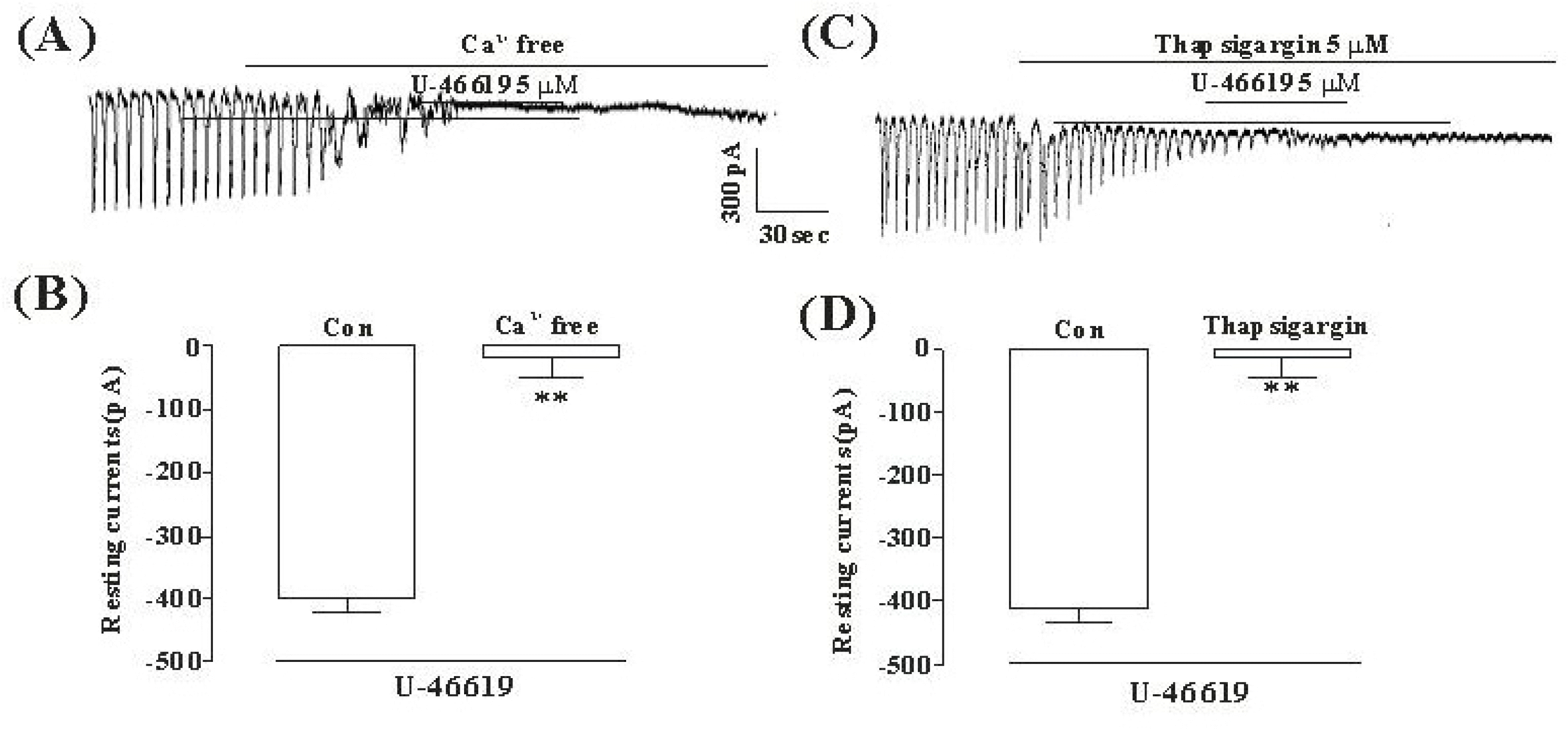
Fig. 4.
The effects of chelerythrine or calphostin C on the TXA2-induced response on pacemaker currents in cultured ICC from mouse small intestine (A), (B) Pacemaker currents of ICC exposed to TXA2 (10 μM) in the presence of chelerythrine (1μM) or calphostin C (0.1μM). In this condition, the TXA2 caused tonic inward currents. The dotted lines indicate the zero current levels. Responses to the TXA2 in the presence of chelerythrine or calphostin C are summarized in (C). Vertical solid line scales amplitude of pacemaker current and horizontal solid line scales duration of recording (s) pacemaker current. The bars represent mean values ± SE.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download