Abstract
Single-channel recordings of TASK-1 and TASK-3, members of two-pore domain K+ channel family, have not yet been reported in dorsal root ganglion (DRG) neurons, even though their mRNA and activity in whole-cell currents have been detected in these neurons. Here, we report single-channel kinetics of the TASK-3-like K+ channel in DRG neurons and up-regulation of TASK-3 mRNA expression in tissues isolated from animals with spinal cord injury (SCI). In DRG neurons, the single-channel conductance of TASK-3-like K+ channel was 33.0±0.1 pS at −60 mV, and TASK-3 activity fell by 65±5% when the extracellular pH was changed from 7.3 to 6.3, indicating that the DRG K+ channel is similar to cloned TASK-3 channel. TASK-3 mRNA and protein levels in brain, spinal cord, and DRG were significantly higher in injured animals than in sham-operated ones. These results indicate that TASK-3 channels are expressed and functional in DRG neurons and the expression level is up-regulated following SCI, and suggest that TASK-3 channel could act as a potential background K+ channel under SCI-induced acidic condition.
REFERENCES
Basso DM., Beattie MS., Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 12:1–21. 1995.

Braughler JM., Hall ED. Effects of multi-dose methylprednisolone sodium succinate administration on injured cat spinal cord neurofilament degradation and energy metabolism. J Neurosurg. 61:290–295. 1984.

Cooper BY., Johnson RD., Rau KK. Characterization and function of TWIK-related acid sensing K+ channels in a rat nociceptive cell. Neuroscience. 129:209–224. 2004.
Farooque M., Hillered L., Holtz A., Olsson Y. Effects of methylprednisolone on extracellular lactic acidosis and amino acids after severe compression injury of rat spinal cord. J Neurochem. 66:1125–1130. 1996.

Holzer P. Acid-sensitive ion channels in gastrointestinal function. Curr Opin Pharmacol. 3:618–625. 2003.

Kang D., Choe C., Kim D. Thermosensitivity of the two-pore domain K+ channels TREK-2 and TRAAK. J Physiol. 564:103–116. 2005.
Kang D., Han J., Talley EM., Bayliss DA., Kim D. Functional expression of TASK-1/TASK-3 heteromers in cerebellar granule cells. J Physiol. 554:64–77. 2004a.

Kang D., Kim D. Single-channel properties and pH sensitivity of two-pore domain K+ channels of the TALK family. Biochem Biophys Res Commun. 315:836–844. 2004.
Kang D., Kim D. TREK-2 (K2P10.1) and TRESK (K2P18.1) are major background K+ channels in dorsal root ganglion neurons. Am J Physiol Cell Physiol. 291:C138–146. 2006.
Kang D., Mariash E., Kim D. Functional expression of TRESK-2, a new member of the tandem-pore K+ channel family. J Biol Chem. 279:28063–28070. 2004b.
Kim D. Fatty acid-sensitive two-pore domain K+ channels. Trends Pharmacol Sci. 24:648–654. 2003.
La JH., Kang D., Park JY., Hong SG., Han J. A novel acid-sensitive K (+) channel in rat dorsal root ganglia neurons. Neurosci Lett. 406:244–249. 2006.
Lang-Lazdunski L., Bachet J., Rogers C. Repair of the descending thoracic aorta: impact of open distal anastomosis technique on spinal cord perfusion, neurological outcome and spinal cord histopathology. Eur J Cardiothorac Surg. 26:351–358. 2004.

Medhurst AD., Rennie G., Chapman CG., Meadows H., Duckworth MD., Kelsell RE., Gloger II., Pangalos MN. Distribution analysis of human two pore domain potassium channels in tissues of the central nervous system and periphery. Brain Res Mol Brain Res. 86:101–114. 2001.

Oh U., Hwang SW., Kim D. Capsaicin activates a nonselective cation channel in cultured neonatal rat dorsal root ganglion neurons. J Neurosci. 16:1659–1667. 1996.

Rau KK., Cooper BY., Johnson RD. Expression of TWIK-related acid sensitive K+ channels in capsaicin sensitive and insensitive cells of rat dorsal root ganglia. Neuroscience. 141:955–963. 2006.
Rush AM., Cummins TR., Waxman SG. Multiple sodium channels and their roles in electrogenesis within dorsal root ganglion neurons. J Physiol. 579:1–14. 2007.

Smith GT. Pharmacological characterization of ionic currents that regulate high-frequency spontaneous activity of electromotor neurons in the weakly electric fish, Apteronotus leptorhynchus. J Neurobiol. 66:1–18. 2006.
Fig. 1.
pH sensitive background K+ channels in DRG neurons. (A) Photomicrograph of DRG neurons cultured for two days in medium containing NGF. These neurons were isolated from postnatal day 1 or 2 (P1-2) rats. Scale bar, 50 μm. (B) The expression of K2P channel mRNA. In the P1~2 rat DRG, PCR products of TASK-1 (702 bp), TASK-2 (441 bp), TASK-3 (517 bp), TREK-1 (361 bp), TREK-2 (493 bp), TRAAK (445 bp), and TRESK (475 bp) were obtained and confirmed by sequencing. (C) Cell-attached patches were formed on DRG neurons, and single-channel openings were recorded at +60 mV and −60 mV at room temperature. Pipette and bath solutions contained 150 mM KCl. Representative traces show five types of single-channel current at +60 mV (upper trace), 0 mV (middle), and −60 mV (lower). The horizontal scale bar indicates 100 ms. The vertical scale bar indicates 2 pA for TRESK and 6 pA for other channels. Bar graphs show the pH sensitivity of each channel (mean±SE, n=4~6). ∗p<0.05 and ∗∗p<0.01 compared to the corresponding control (pH 7.3).
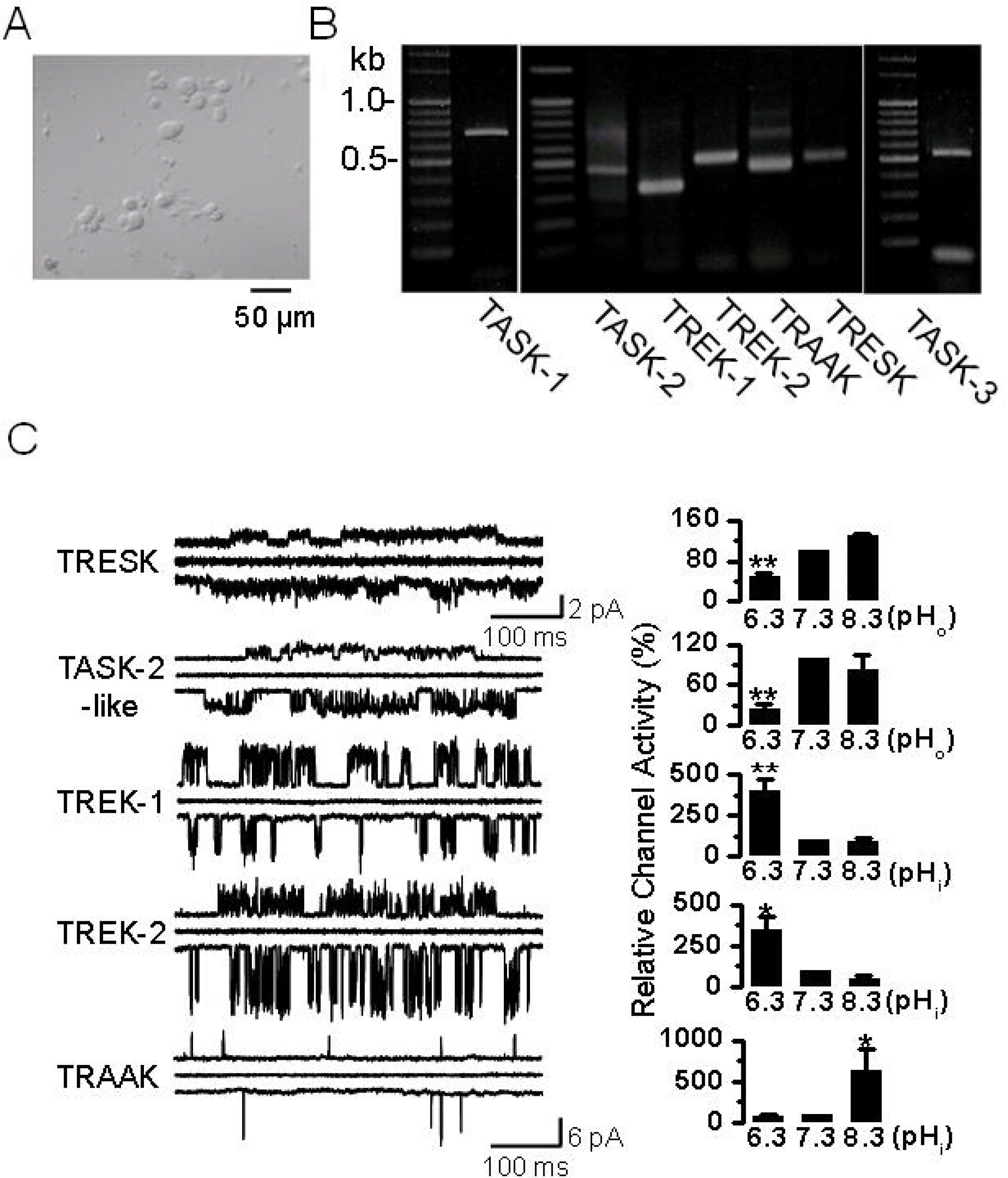
Fig. 2.
Single-channel kinetics of TASK-3-like K+ channels in DRG neurons. (A) Cell-attached patches were formed, and single-channel openings were recorded at the different membrane potentials, noted on the left. Pipette and bath solutions contained 150 mM KCl. (B) Histograms of open time duration (left) and amplitude (right) of TASK-3-like K+ channel were obtained from openings at −60 mV. They were fitted by single exponential and Gaussian functions, respectively. (C) Single-channel amplitudes were determined from the amplitude histogram for each membrane potential in order to construct the current-voltage relationship. Each point represents mean±SD of four repeated experiments. (D) The pHo sensitivity of single-channel currents from TASK-3-like K+ channels in DRG neurons. Single-channel currents from outside-out patches are shown as inward currents recorded at −60 mV when KCl concentration was 150 mM on both sides of the patch. Typical channel openings from three independent experiments are shown. The bar graph shows the effects of pHo changes on TASK-3-like K+ channel activity. Each bar represents mean±SD of three repeated experiments. The asterisk indicates significant difference from the control value at pH 7.3 (p<0.05).
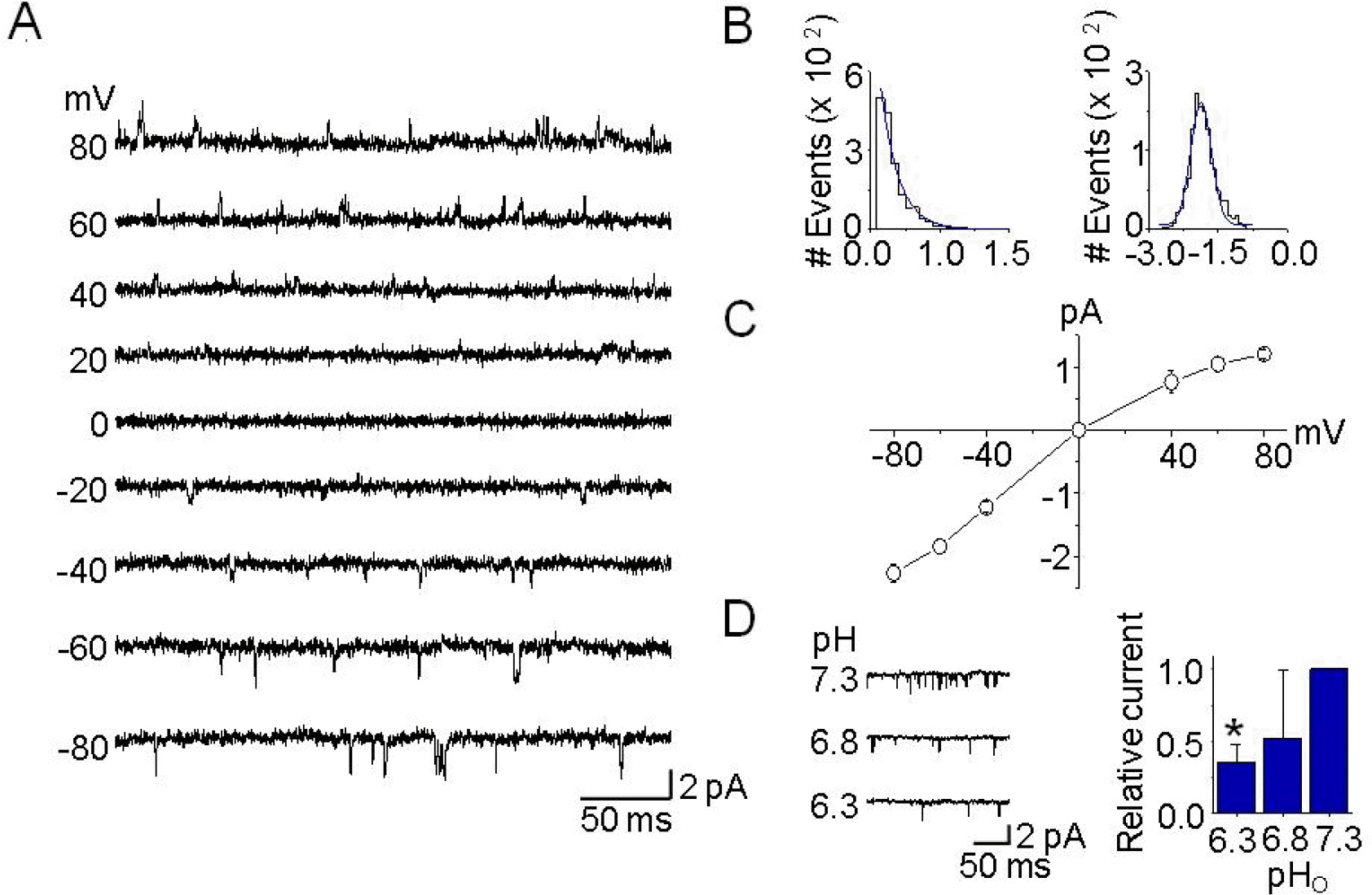
Fig. 3.
Up-regulation of TASK-3 mRNA and protein expression after spinal cord injury. (A) A photograph of rats with a T9 spinal segment injury. These rats exhibited paralysis of the hind legs. (B) The BBB open-field locomotor rating scores show spontaneous partial recovery of motor function after SCI (n=10 animals per group). Data points represent mean±SD. (C) Changes in TASK-3 mRNA expression in SCI animals. PCR was conducted on cDNA templates obtained from brain, spinal cord, and DRG of SCI and sham-operated animals at 48 hours after injury. The bar graph summarizes the levels of TASK-3 mRNA expression which changed after SCI. Each bar represents mean±SD of three repeated experiments. Asterisks indicate significant difference from the corresponding control value (sham-operated groups, p <0.05). (D) Western blot analysis in DRG. TASK-3 protein level increased in DRG obtained from SCI animal at 48 hours after injury. The bar graph shows the up-regulation of TASK-3 protein expression in DRG obtained from SCI animals. Each bar represents mean±SD of three repeated experiments. Asterisks (∗∗) indicate significant difference from the corresponding control value (sham-operated groups, p<0.01).
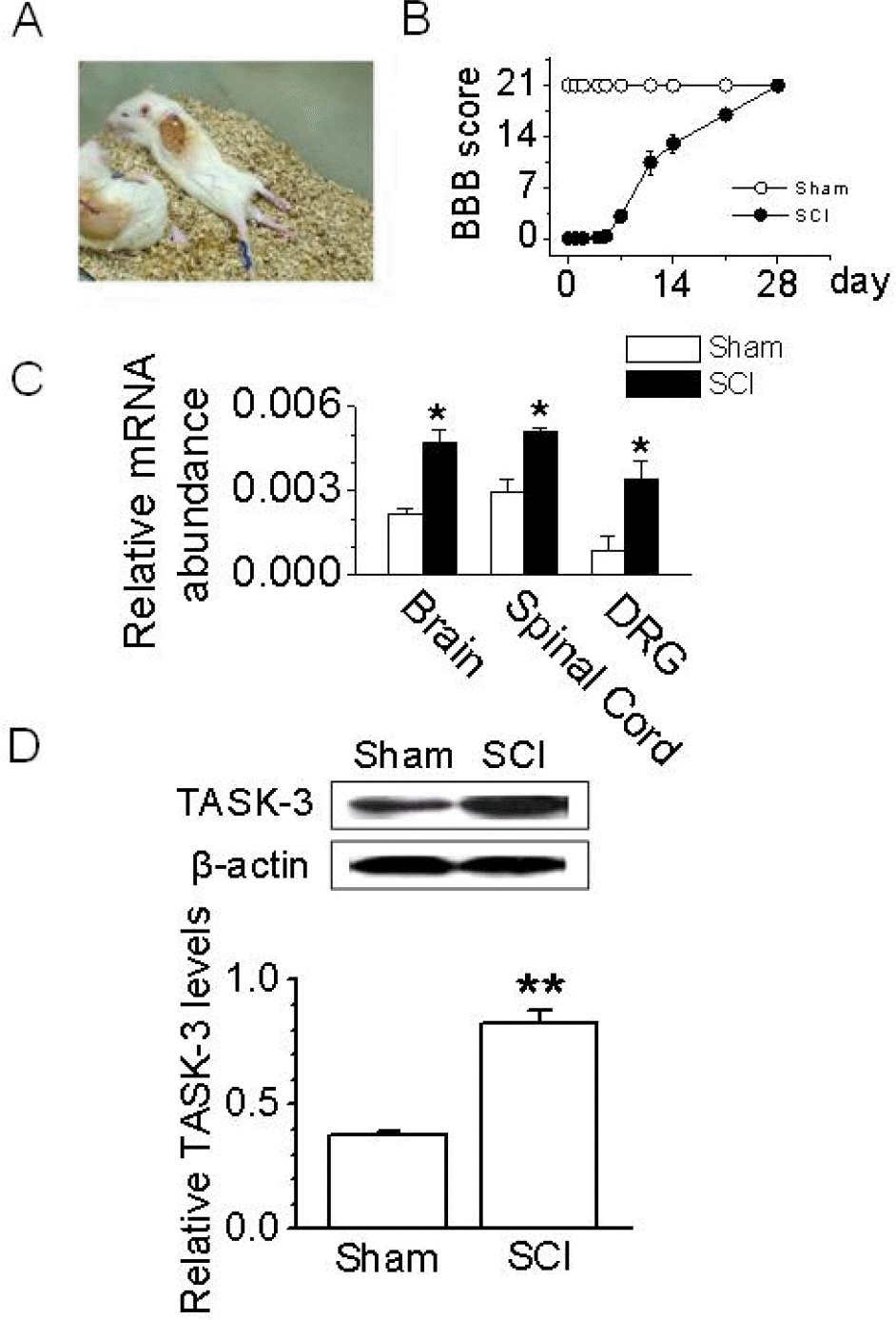
Table 1.
Primer sequences used for RT-PCR and real-time PCR




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download