Abstract
The layers of keratinocytes form an acid mantle on the surface of the skin. Herein, we investigated the effects of acidic pH on the membrane current and [Ca2+]c of human primary keratinocytes from foreskins and human keratinocyte cell line (HaCaT). Acidic extracellular pH (pHe≤ 5.5) activated outwardly rectifying Cl current (ICl,pH) with slow kinetics of voltage-dependent activation. ICl,pH was potently inhibited by an anion channel blocker 4,4‘-diisothiocyanostilbene-2,2’-disulphonic acid (DIDS, 73.5% inhibition at 1 μM). ICl,pH became more sensitive to pHe by raising temperature from 24°C to 37°C. HaCaT cells also expressed Ca2+-activated Cl− current (ICl,Ca), and the amplitude of ICl,Ca was increased by relatively weak acidic pHe (7.0 and 6.8). Interestingly, the acidic pHe (5.0) also induced a sharp increase in the intracellular [Ca2+] (Δ [Ca2+]acid) of HaCaT cells. The Δ [Ca2+]acid was independent of extracellular Ca2+, and was abolished by the pretreatment with PLC inhibitor, U73122. In primary human keratinocytes, 5 out of 28 tested cells showed Δ[Ca2+]acid. In summary, we found ICl,pH and Δ[Ca2+]acid in human keratinocytes, and these ionic signals might have implication in pathophysiological responses and differentiation of epidermal keratinocytes.
REFERENCES
Auzanneau C., Thoreau V., Kitzis A., Becq F. A novel voltage-dependent chloride current activated by extracellular acidic pH in cultured rat Sertoli cells. J Biol Chem. 278:19230–19236. 2003.

Behne MJ., Meyer JW., Hanson KM., Barry NP., Murata S., Crumrine D., Clegg RW., Gratton E., Holleran WM., Elias PM., Mauro TM. NEH1 regulates the stratum corneum permeability barrier homeostasis. J Biol Chem. 277:47299–47406. 2002.
Boyce ST., Ham RG. Calcium-regulated differentiation of normal human epidermal keratinocytes in chemically defined clonal culture and serum-free serial culture. J Invest Dermatol. 81(1 Suppl):33s–40s. 1983.

Elias PM., Ahn SK., Brown BE., Crumrine D., Feingold KR. Origin of the epidermal calcium gradient: regulation by barrier status and role of active vs passive mechanisms. J Invest Dermatol. 119:1269–1274. 2002.

Fluhr JW., Kao J., Jain M., Ahn SK., Feingold KR., Elias PM. Generation of free fatty acids from phospholipids regulates stratum corneum acidification and integrity. J Invest Dermatol. 117:44–51. 2001.

Fluhr JW., Mao-Qiang M., Brown BE., Hachem J-P., Moskowitz DG., Demerjian M., Haftek M., Serre G., Crumrine D., Mauro TM., Elias PM., Feingold KR. Functional consequences of a neutral pH in neonatal rat stratum corneum. J Invest Dermatol. 123:140–151. 2004.

Frosch P., Kligman AM. Method for appraising the sting capacity of topically applied substances. J Soc Cosmetic Chem. 28:197–209. 1977.
Hachem J-P., Crumrine D., Fluhr J., Brown BE., Feingold KR., Elias PM. pH directly regulates epidermal permeability barrier homeostasis, and stratum corneum integrity/cohesion. J Invest Dermatol. 121:345–353. 2003.

Hirayama Y., Kuruma A., Hiraoka M., Kawano S. Calcium-activated Cl− current is enhanced by acidosis and contributes to the shortening of action potential duration in rabbit ventricular myocytes. Jpn J Physiol. 52:293–300. 2002.
Ishii S., Kihara Y., Shimizu T. Identification of T cell death-associated gene 8 (TDAG8) as a novel acid sensing G-protein-coupled receptor. J Biol Chem. 280:9083–9087. 2005.

Koegel H., Alzheimer C. Expression and biological significance of Ca2+-activated ion channels in human keratinocytes. FASEB J. 15:145–154. 2001.
Lambert S., Oberwinkler J. Characterization of a proton-activated, outwardly rectifying anion channel. J Physiol. 567:191–213. 2005.

Lang F., Föller M., Lang KS., Lang PA., Ritter M., Gulbins E., Vereninov A., Huber SM. Ion channels in cell proliferation and apoptotic cell death. J Memb Biol. 205:147–157. 2005.

Lehen'kyi V., Beck B., Polakowska R., Charveron M., Bordat P., Skryma R., Prevarskaya N. TRPV6 is a Ca2+ entry channel essential for Ca2+-induced differentiation of human keratinocytes. J Biol Chem. 282:22582–22591. 2007.
Ludwig M-G., Vanek M., Guerini D., Gasser JA., Jones CE., Junker W., Hofstetter H., Wolf RM., Seuwen K. Proton-sensing G-protein coupled receptors. Nature. 425:93–98. 2003.
Menkin V. Chemical mediators in relation to cytologic constituents in inflammation. Am J Pathol. 34:921–941. 1958.
Menon GK., Elias PM., Lee SH., Feingold KR. Localization of calcium in murine epidermis following disruption and repair of the permeability barrier. Cell Tissue Res. 270:503–512. 1992.

Murakami N., Yokomizo T., Okuno T., Shimizu T. G2A is a protonsensing G-protein-coupled receptor antagonized by lysophosphatidylcholine. J Biol Chem. 279:42484–42491. 2004.

Nilius B., Droogmans G. Amazing chloride channels: an overview. Acta Physiol Scand. 177:119–147. 2003.

Nobles M., Higgins CF., Sardini A. Extracellular acidification elicits a chloride current that shares characteristics with ICl(swell). Am J Physiol Cell Physiol. 287:C1426–C1435. 2004.
Okada Y., Maeno E., Shimizu T., Manabe K., Mori S., Nabekura T. Dual roles of plasmalemmal chloride channels in induction of cell death. Pflugers Arch. 448:287–295. 2004.

Silver RB. Ratio imaging: practical considerations for measuring intracellular calcium and pH in living tissue. Methods Cell Biol. 56:237–251. 1998.
Smith JB., Dwyer SD., Smith L. Lowering extracellular pH evokes inositol polyphosphate formation and calcium mobilization. J Biol Chem. 264:8723–8728. 1989.

Tu C-L., Chang W., Bikle DD. The extracellular calcium-sensing receptor is required for calcium-induced differentiation in human keratinocytes. J Biol Chem. 276:41079–41085. 2001.

Wang H-Y., Shimizu T., Numata T., Okada Y. Role of acid-sensitive outwardly rectifying anion channels in acidosis-induced cell death in human epithelial cells. Pflugers Arch. 454:223–233. 2007.

Willis CM., Shaw S., Lacharriere ODE., Baverel M., Reiche L., Jourdain R., Bastien P., Wilkinson J. Sensitive skin: an epidemiological study. Br J Dermatol. 145:258–263. 2001.

Fig. 1.
Activation of outwardly rectifying Cl current by acidic pHe. Representative current traces obtained from primary keratinocytes (A) and HaCaT cells (B) by steplike pulses. The membrane voltage was held at −40 mV, and incremental step-like pulses from −100 to 100 mV (20 mV intervals, 400 ms duration, see inset). The currents (B-a) were obtained with Nagluconate solution in the bath (pH 4.2). (B-b) Representative current traces obtained by step-like pulses under different pHe. The current to voltage relations (I-V curves), measured at the end of each pulse, are shown in right panels. Step like pulses were applied in different conditions of extracellular anions (pH 4.2 Br−, and I−). Current traces presented in (C) were obtained from the same cell, respectively. I-V curves of ICl,pH obtained in NaCl, NaBr, and NaI solutions are shown in the right panel of (C) Each symbol represents mean±SEM of current amplitudes normalized to cell capacitance (pA/pF).
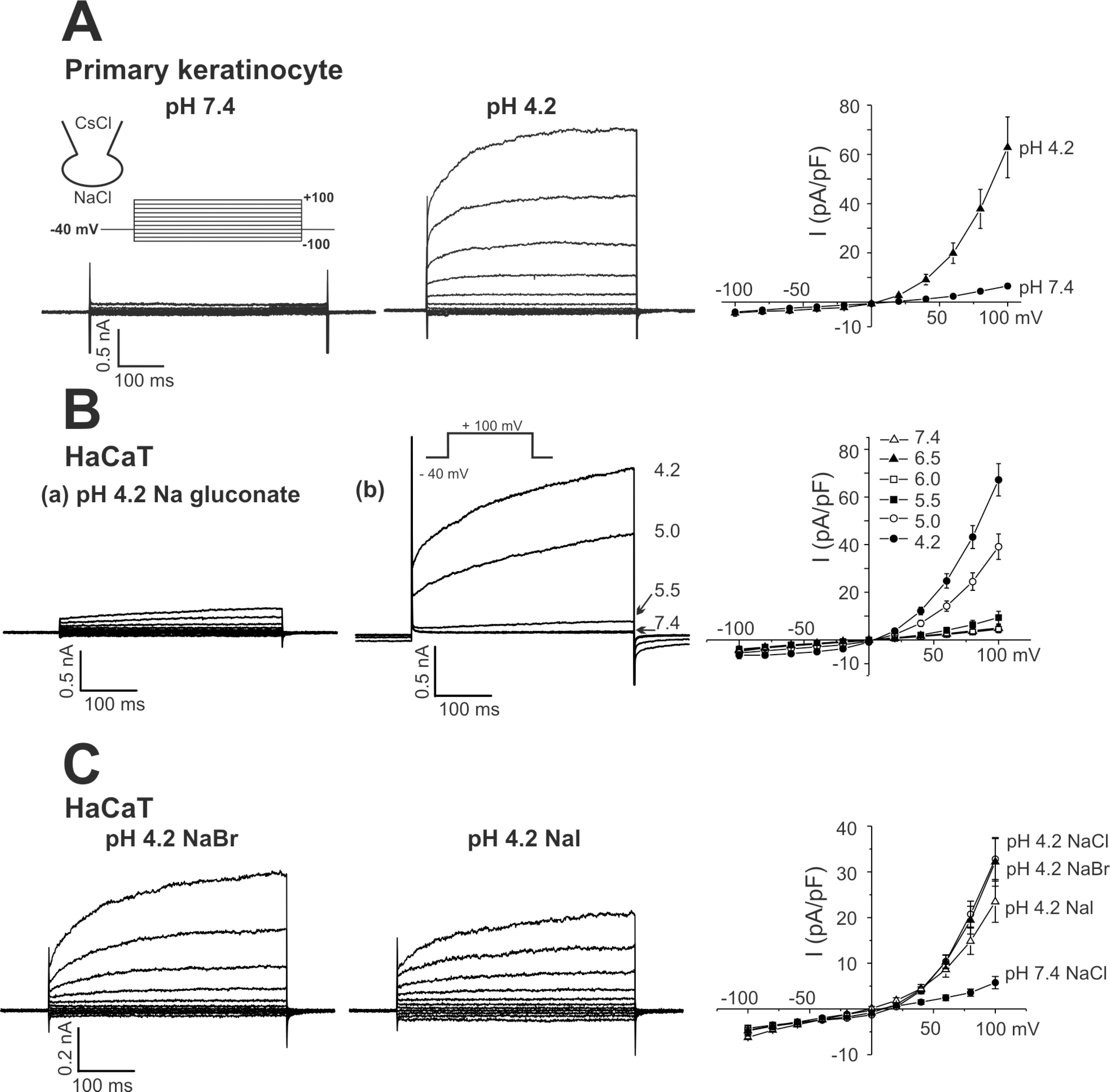
Fig. 2.
Effects of anion channel blockers on ICl,pH. (A, B) Representative current traces obtained by step-like pulses, same as in Fig. 1. ICl,pH was activated by pHe 5.0, and DIDS (1μM) or niflumic acid (NFA, 100μM) was added (right panels). (C, D) I-V curves of ICl,pH activated by pHe 5.0 and effects of DIDS (0.3μM and 1μM) or niflumic acid (30μM and 100μM). Each symbol represents mean±SEM (n=5, respectively) of current amplitudes normalized to the cell capacitance (pA/pF).
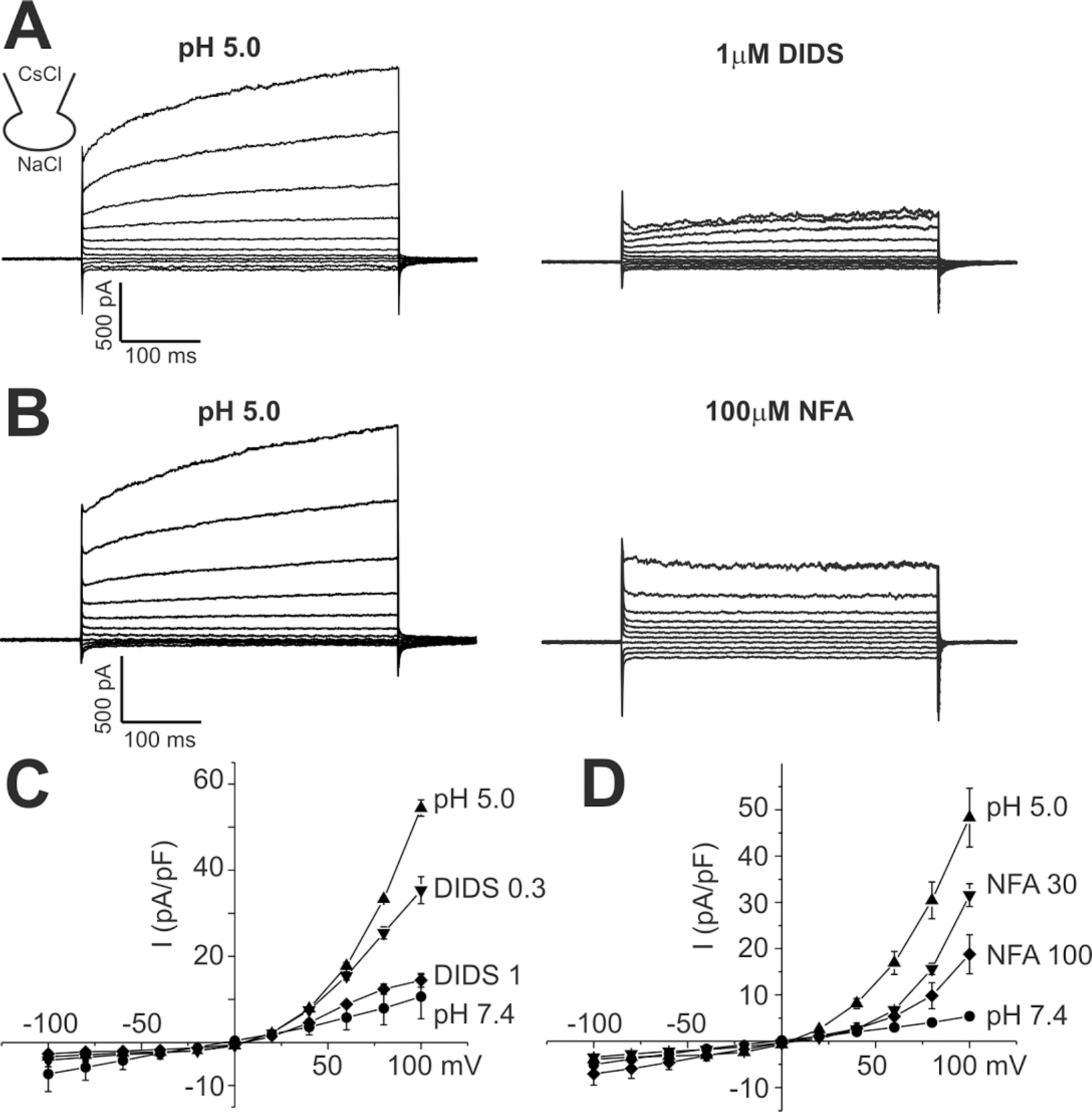
Fig. 3.
Effects of bath temperature on ICl,pH activation in HaCaT cells. (A) Representative current traces obtained by step like pulses, same as in Fig. 1. Current traces at pH 7.4, 5.5, and 5.0 were recorded under room temperature (24°C, left panels) and 37°C (right panels). The raise of temperature alone had insignificant effect on membrane conductance, whereas substantial increase was shown at pH 5.5. At pH 5.0, high temperature increased the activation kinetics with similar peak amplitude. All current trances shown were obtained from the same cell. (B) Mean amplitudes of normalized ICl,pH (pA/pF) measured at 100 mV under different pHe and temperature were compared (n=8) ∗indicate p-value<0.05.
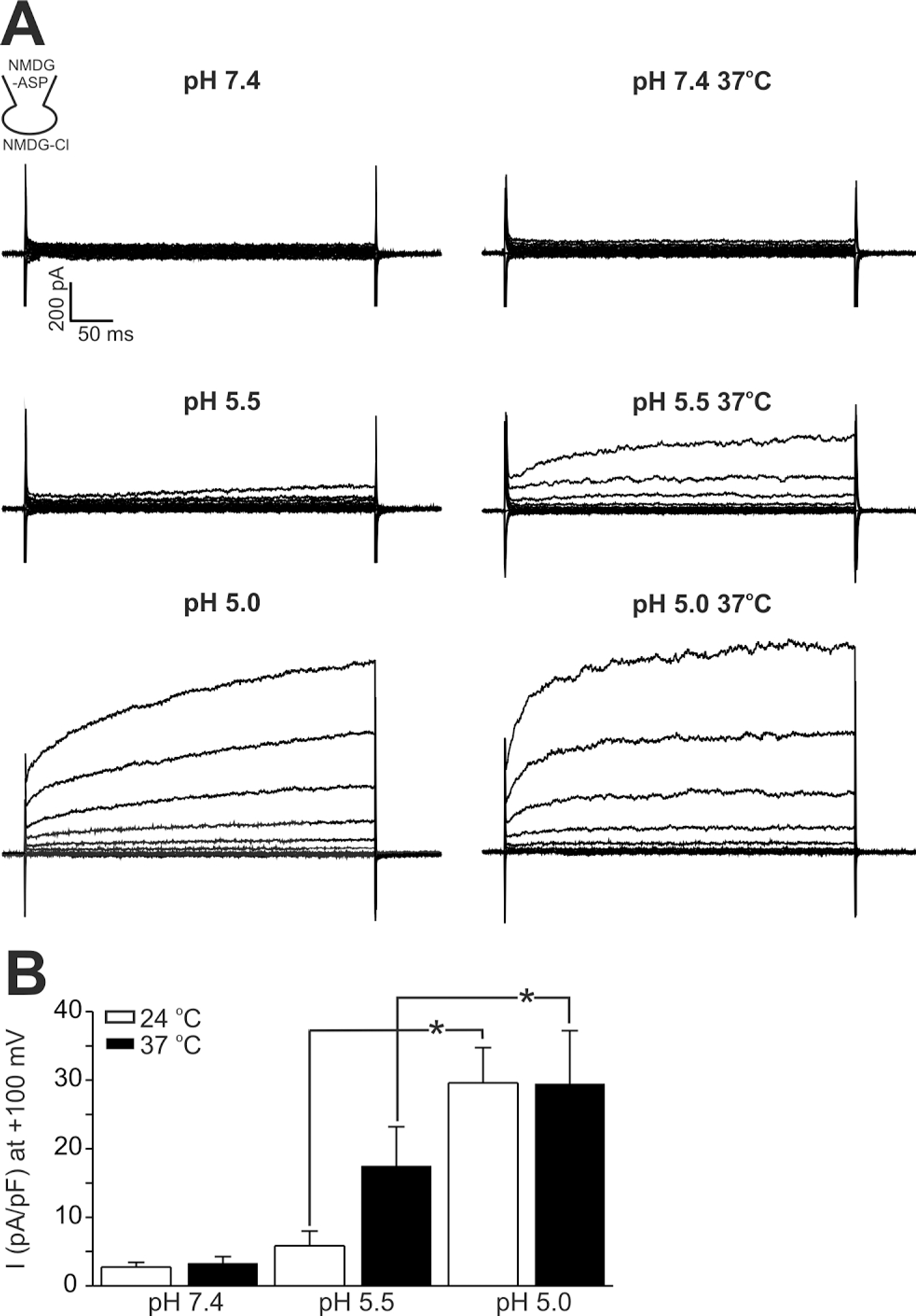
Fig. 4.
Ca2+-activated Cl− current (ICl,Ca) of HaCaT cells and effects of acidic pHe. (A) Outward currents with slowly activating kinetics were induced by dialyzing cells against Ca2+-clamped CsCl solution (see inset). Lowering pHe to 7.0 and 6.8 increased the amplitude of outward current. After testing pHe effects, the inhibition of outward current by 500 μM DIDS was confirmed at pHe 6.8. (B) Summary of the effects of pHe on ICl,Ca of HaCaT cells. In each cell, the current amplitudes measured at the end of step-pulse were normalized to the control amplitude (pHe 7.4), and mean±SEM values were plotted (n=8). ∗indicate p-value<0.05.
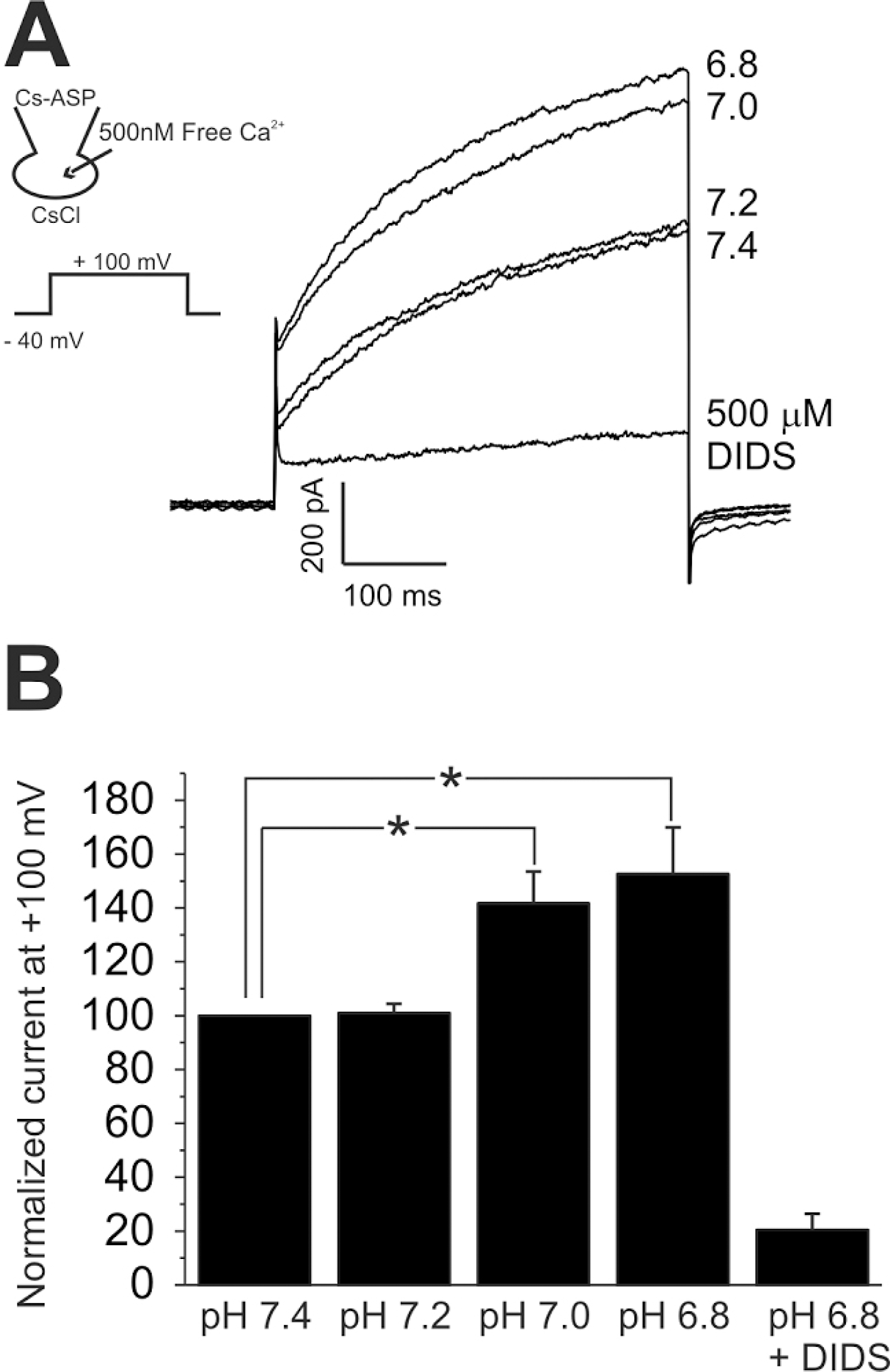
Fig. 5.
Acidic pHe-induced increase of [Ca2+]c (Δ[Ca2+]acid). (A, B) Representative traces of the fluorescence ratio (F340/380) and the responses of HaCaT (A) and primary keratinyocyte (B) to pHe 5.0 and ATP (100 μM). The Δ[Ca2+]acid was persistent in the absence of extracellular Ca2+ (A). (C, D) Inhibition of Δ[Ca2+]acid by PLC inhibitor (U73122, 2 μM)), whereas no effect of the negative analogue (U73343, 2 μM).
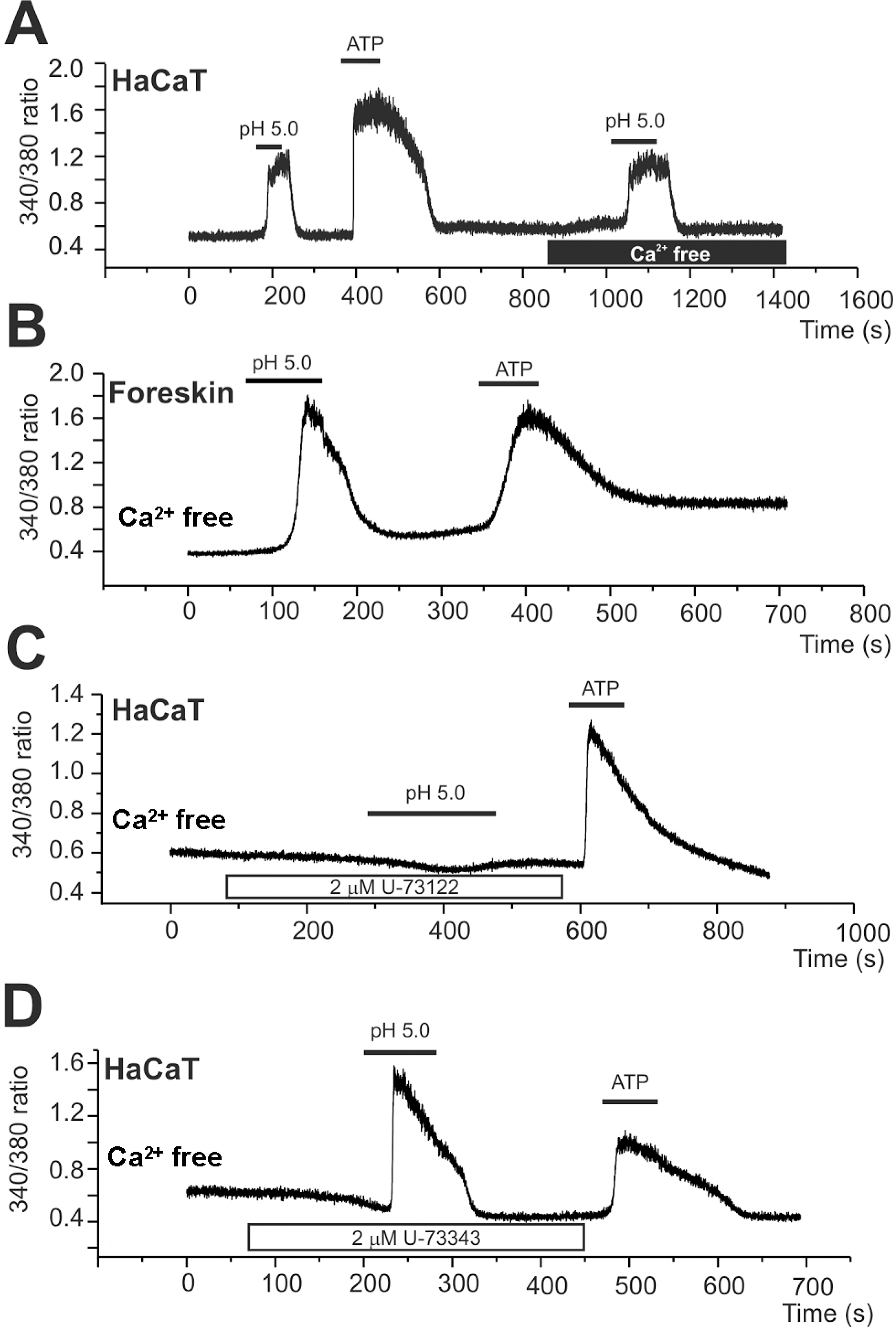
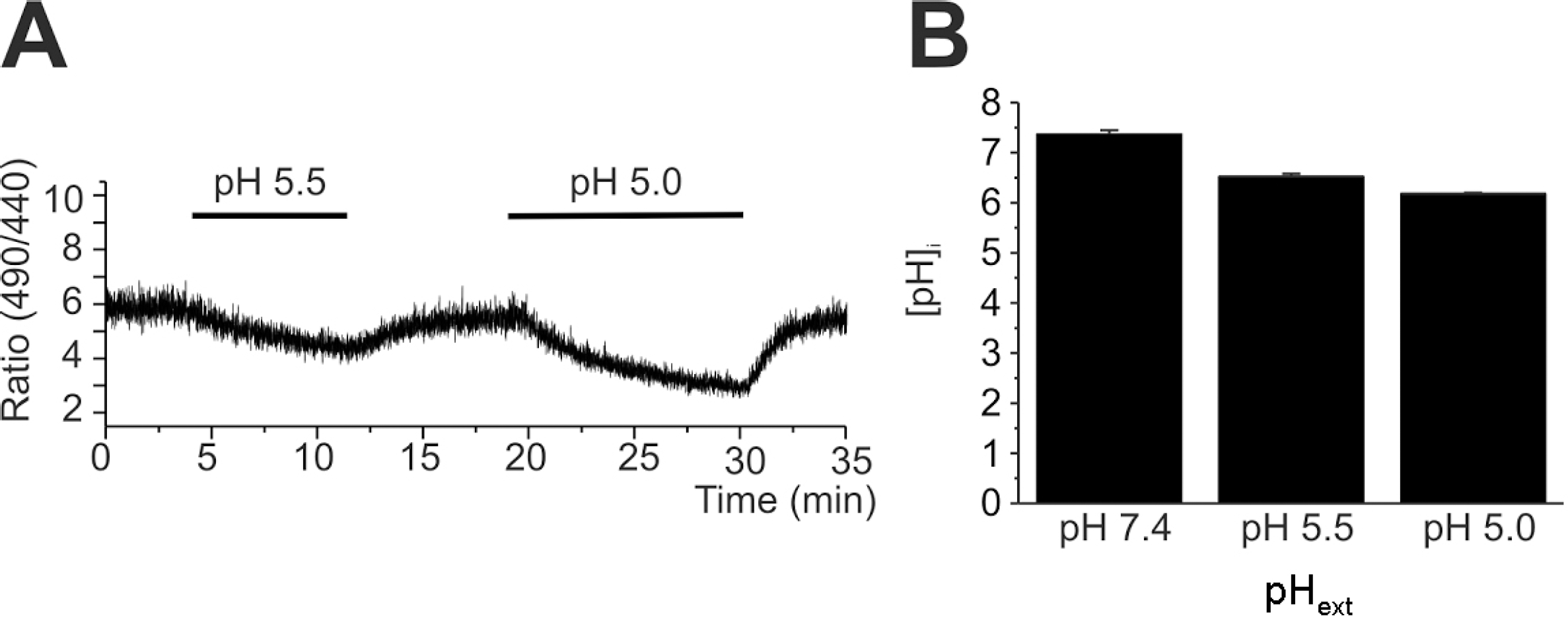
Fig. 6.
Slow decrease of intracellular pH (pHi) in response to extracellular acidification. (A) A representative trace of BCECF emission ratio (F490/440) measured in HaCaT cell. pHe was changed from 7.4 to 5.5. or to 5.0 as indicated. (B) Summary of calibrated pHi (mean± SEM, n=8) of control (pHe 7.4) and 10 min after pHe changes (5.5 and 5.0).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download