Abstract
The effects of (–)-epigallocatechin gallate (EGCG) on pacemaker activities of cultured interstitial cells of Cajal (ICC) from murine small intestine were investigated using whole-cell patch-clamp technique at 30°C and Ca2+ image analysis. ICC generated spontaneous pacemaker currents at a holding potential of −70 mV. The treatment of ICC with EGCG resulted in a dose-dependent decrease in the frequency and amplitude of pacemaker currents. SQ-22536, an adenylate cyclase inhibitor, and ODQ, a guanylate cyclase inhibitor, did not inhibit the effects of EGCG. EGCG-induced effects on pacemaker currents were not inhibited by glibenclamide, an ATP-sensitive K+ channel blocker and TEA, a Ca2+-activated K+ channel blocker. Also, we found that EGCG inhibited the spontaneous [Ca2+]i oscillations in cultured ICC. In conclusion, EGCG inhibited the pacemaker activity of ICC and reduced [Ca2+]i oscillations by cAMP-, cGMP-, ATP-sensitive K+ channel-independent manner.
REFERENCES
Alvarez E., Campos-Toimil M., Hustiniano-Basaran H., Lugnier C., Orallo F. Study of the mechanisms involved in the vasorelaxation induced by (–)-epigallocatechin-3-gallate in rat aorta. Br J Pharm. 147:269–280. 2006.
Alvarez-Castro E., Campos-Toimil M., Orallo F. (–)-epigallocatechin-3-gallate induces contraction of the rat aorta by a calcium influx-dependent mechanism. Naunyn Schmiedebergs Arch Pharmacol. 369:496–506. 2004.
Aoyama M., Yamada A., Wang J., Ohya S., Furuzono S., Goto T., Hotta S., Ito Y., Matsubara T., Shimokata K., Chen SR., Imaizumi Y., Nakayama S. Requirement of ryanodine receptors for pacemaker Ca2+ activity in ICC in HEK293 cells. J Cell Sci. 117:2813–2825. 2004.
Baek WK., Jang BC., Lim JH., Kwon TK., Lee HY., Cho CH., Kim DK., Shin DH., Park JG., Lim JG., Bae JH., Bae JH., Yoo SK., Park WK., Song DK. Inhibitory modulation of ATP-sensitive potassium channels by gallate-ester moiety of (–)-epigallocatechin-3-gallate. Biochem Pharm. 70:1560–1567. 2005.

Ceregrzyn M., Kuwahara A. The effect of epigallocatechin gallate on intestinal motility in mice. Environ Health Prev Med. 8:47–51. 2003.

Chen ZY., Law WI., Yao XQ., Lau CW., Ho WK., Huang Y. Inhibitory effects of purified green tea epicatechins on contraction and proliferation of arterial smooth muscle cells. Acta Pharmacologica Sinica. 21:835–840. 2000.
Choi S., Chang IY., Yeum CH., You HJ., Park JS., Jeong HS., So I., Kim KW., Jun JY. Activating of ATP-dependent K+ channels comprised of Kir 6.2 and SUR2B by PGE2 through EP2 receptor in cultured interstitial cells of Cajal from murine small intestine. Cell Physiol Biochem. 18:187–198. 2006.
Di Carlo G., Autore G., Izzo AA., Maiolino P., Mascolo N., Viola P., Diurno MV., Capasso F. Inhibition of intestinal motility and secretion by flavonoids in mice and rats: structure-activity relationships. J Pharm Pharmacol. 45:1054–1059. 1993.

Graham HN. Green tea composition, consumption, and polyphenol chemistry. Prevent Med. 21:334–350. 1992.

Hara Y. Green Tea; Health benefits and applications. Marcel Dekker, Inc;New York: 2001.
Homma T., Hirai K., Hara Y., Katayama Y. Tea catechin, (–)-epigallocatechin gallate, causes membrane depolarizations of myenteric neurons in the guinea-pig small intestine. Neurosci Lett. 309:93–96. 2001.

Huang Y., Zhang A., Lau CW., Chen ZY. Vasorelaxant effects of purified green tea epicatechin derivatives in rat mesenteric artery. Life Sci. 63:275–283.

Huizinga JD., Thuneberg L., Kluppel M., Malysz J., Mikkelsen HB., Bernstein A. W/kit gene required for intestinal pacemaker activity. Nature. 373:347–352. 1995.
Jayaraj AP., Lewin MR., Tovey FI., Kitler ME., Clark CG. The protective effect of Meciadanol (O-methyl-3(+)-catechin) on experimental ulceration. Eur J Pharmacol. 147:265–271. 1988.

Koh SD., Sanders KM., Ward SM. Spontaneous electrical rhythmicity in cultured interstitial cells of Cajal from the mouse small intestine. J Physiol. 513:203–213. 1998.
Katayama Y., Homma T., Hara Y., Hara Y., Hirai K. Tea catechin, (–)-epigallocatechin gallate, facilitates cholinergic ganglion transmission in the myenteric plexus of the guinea-pig small intestine. Neurosci Lett. 319:63–66. 2002.

Konturek SJ., Kitler ME., Brzozowski T., Radecki T. Gastric protection by meciadanol. Dig Dis Sci. 31:847–852. 1986.

Matsuzaki T., Hara Y. Antioxidative activity of tea leaf catechins. Nippon Nogeikagaku Kaishi. 59:129–134. 1985.

Minowa T., Miwa S., Kobayashi S., Enoki T., Zhang XF., Komuro T., Iwamuro Y., Masaki T. Inhibitory effect of nitrovasodilators and cyclic GMP on ET-1-activated Ca2+ permeable nonselective cation channel in rat aortic smooth muscle cells. Br J Pharm. 271:515–522. 1990.
Orallo F. Regulation of cytosolic calcium levels in vascular smooth muscle. Pharm Therap. 69:153–171. 1996.

Orlov SN., Tremblay J., Hamet P. cAMP signaling inhibits dihydropyridine-sensitive Ca2+ influx in vascular smooth muscle cells. Hypertension. 27:774–780. 1996.
Park CG., Kim MY., Kim JS., Choi S., Yeum CH., Parajuli SP., Park JS., Jeong HS., So I., Kim KW., Jun JY. Inhibition of pacemaker currents by nitric oxide via activation of ATP-sensitive K+ channels in cultured interstitial cells of Cajal from mouse small intestine. Naunyn-Schmiedeberg Arch Pharmacol. 376:175–184. 2007.
Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 111:492–515. 1996.

Shuttleworth CW., Xue C., Ward SM., de Vent J., Sanders KM. Immunohisochemical localization of 3',5'-cyclic guanosine monophosphate in the canine proximal colon: response to nitric oxide and electrical stimulation of enteric inhibitory neurons. Neuroscience. 56:513–522. 1993.
Suzuki H., Takano H., Yamamoto Y., Komuro T., Saito M., Kato K., Mikoshiba K. Properties of gastric smooth muscles obtained from mice which lack inositol triphosphate receptor. J Physiol. 525:105–111. 2000.
Thomsen L., Robinson TL., Lee JCF., Farraway L., Hughes MJH., Andrews DW., Huizinga JD. Interstitial cells of Cajal generate a rhythmic pacemaker current. Nature Med. 4:848–51. 1998.
Thuneberg L. Interstitial cells of Cajal: Intestinal pacemakers? Adv Anat Embryol Cell Biol. 71:1–130. 1982.
Tokutomi N., Maeda H., Tokutomi Y., Sato D., Sugita M., Nishikawa S., Nishikawa S., Nakao J., Imamura T., Nishi K. Rhythmic Cl-current and physiological roles of the intestinal c-kit-positive cells. Pflugers Arch. 431:169–177. 1995.
Viswanathan S., Thirugnana Sambantham P., Bapna JS., Kameswaran L. Flavonoid-induced delay in the small intestinal transit: possible mechanism of action. Arch Int Pharmacodyn Ther. 270:151–157. 1984.
Fig. 1.
Effects of catechins on pacemaker currents recorded in cultured ICC from mouse small intestine (A) Pacemaker currents of ICC recorded at a holding potential of −70 mV, when exposed to polyphenon 60 (200μM). (B) Pacemaker currents of ICC recorded at a holding potential of −70 mV when exposed to epicatechin gallate (200μM). (C) Pacemaker currents of ICC recorded at a holding potential of −70 mV which were exposed to epigallocatechin gallate (200μM). Vertical solid line scales amplitude of pacemaker current and horizontal solid line scales duration of recording (s) pacemaker currents. ECG: (–)-epicatechin gallate, EGCG: (–)-epigallocatechin gallate.
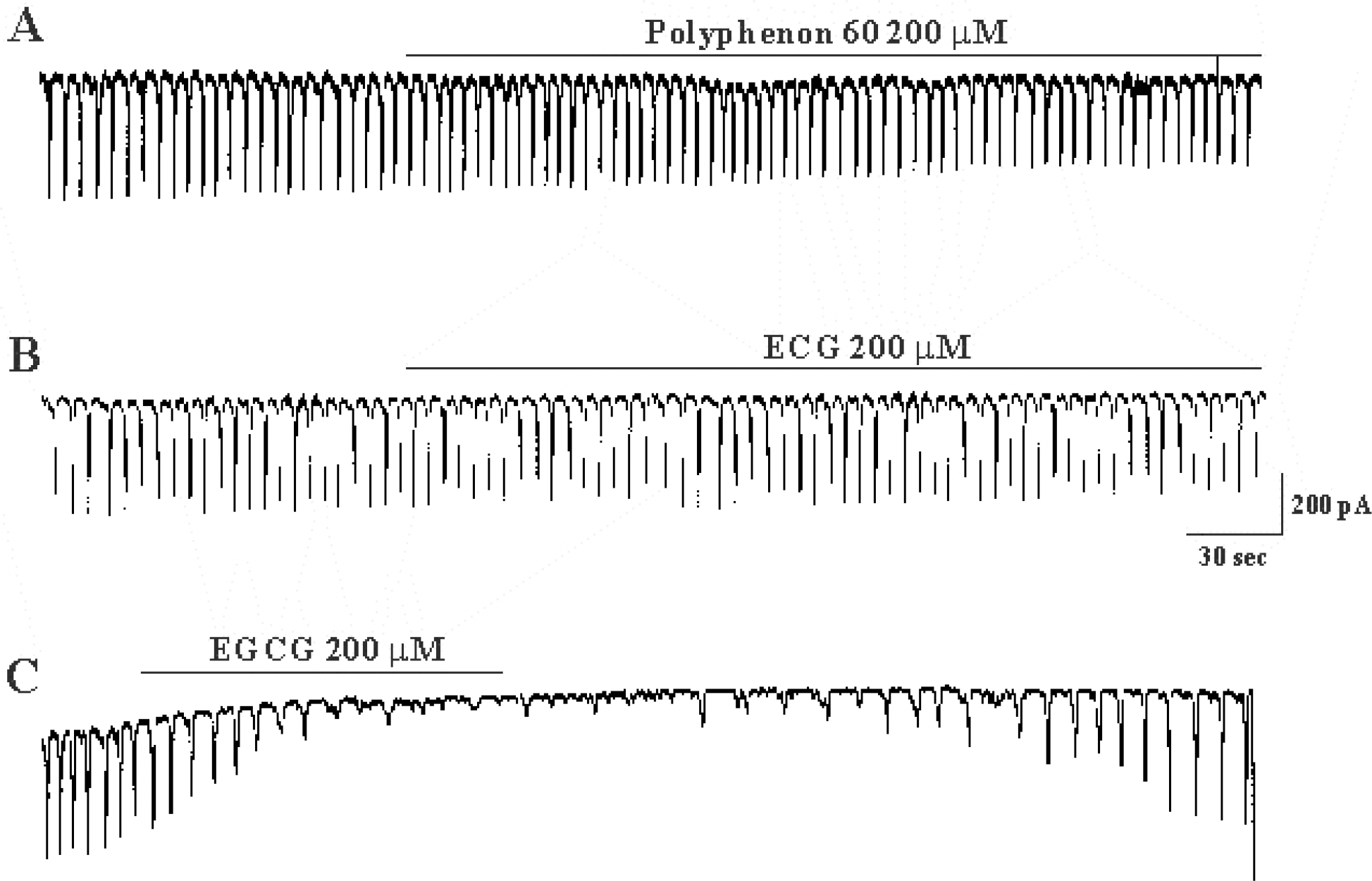
Fig. 2.
Dose-dependent effects of (–)-epigallocatechin gallate on pacemaker currents in cultured ICC of mouse small intestine. (A), (B), and (C) show pacemaker currents of ICC exposed to (–)-epigallocatechin gallate (50, 100, or 200 μM respectively) at a holding potential of −70 mV. Vertical solid line scales amplitude of pacemaker current and horizontal solid line scales duration of recording (s) pacemaker currents. (–)-epigallocatechin gallate at 100 and 200 μM concentrations inhibited spontaneous pacemaker currents of ICC. (D), (E), and (F) summarize the inhibitory effects of (–)-epigallocatechin gallate on pacemaker currents in ICC. Bars represents means±SE (n=5/group). ∗Asterisks mean significantly different from the controls (p<0.05). EGCG: (–)-epigallocatechin gallate, CON: control.
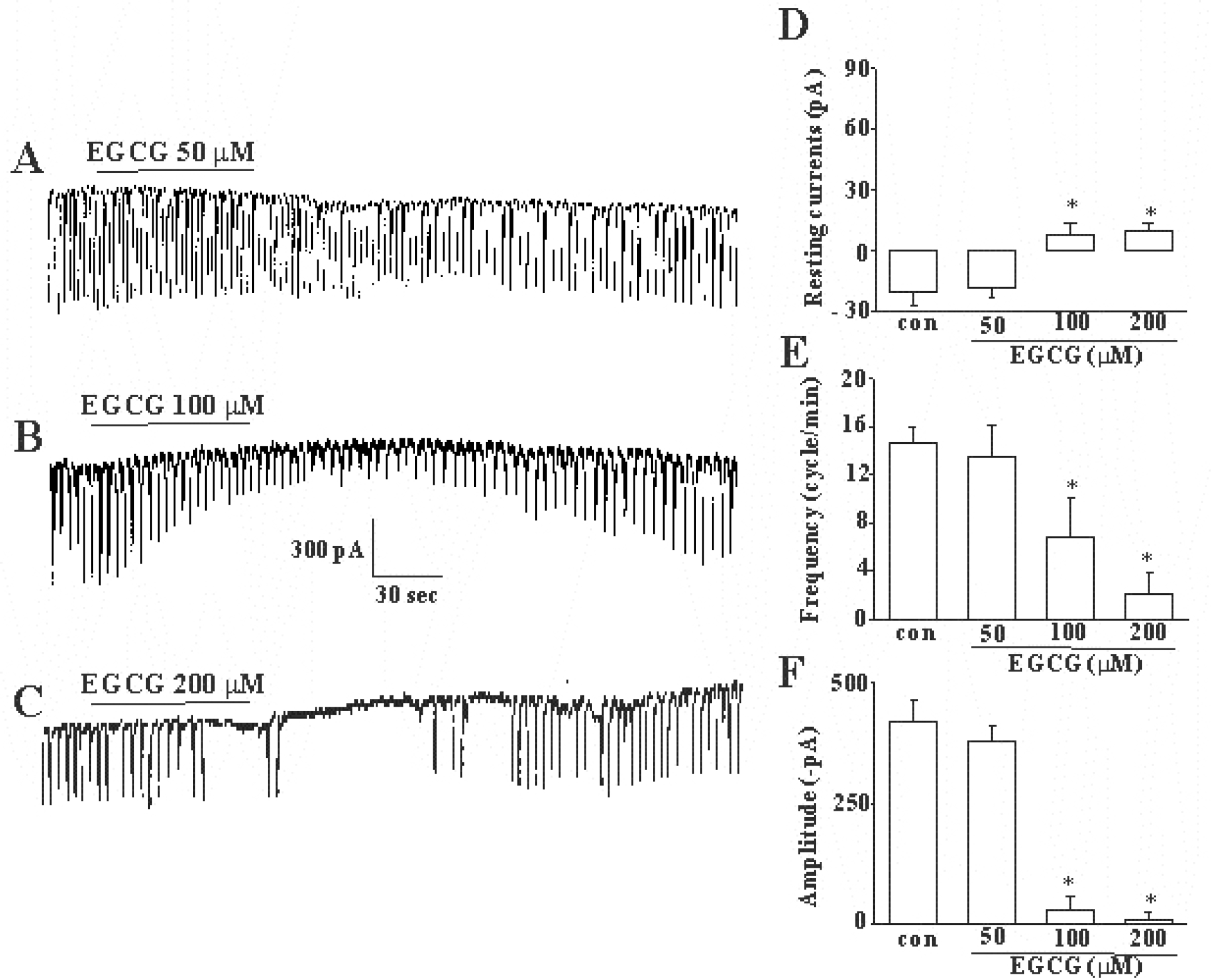
Fig. 3.
Effects of SQ-22536, an inhibitor of adenylate cyclase, and ODQ, an inhibitor of guanylate cyclase, on (–)-epigallocatechin gallate-induced response in cultured ICC from mouse small intestine. (A) Pretreatment with SQ-22536 (10μM) did not affect the inhibitory effects of (–)-epigallocatechin gallate (200μM) on spontaneous inward currents. (B) Pretreatment with ODQ (10μM) did not affect the inhibitory effects of (–)-epigallocatechin gallate (200μM) on spontaneous inward currents. Vertical solid line scales amplitude of pacemaker current and horizontal solid line scales duration of recording (s) pacemaker currents. EGCG: (–)-epigallocatechin gallate.
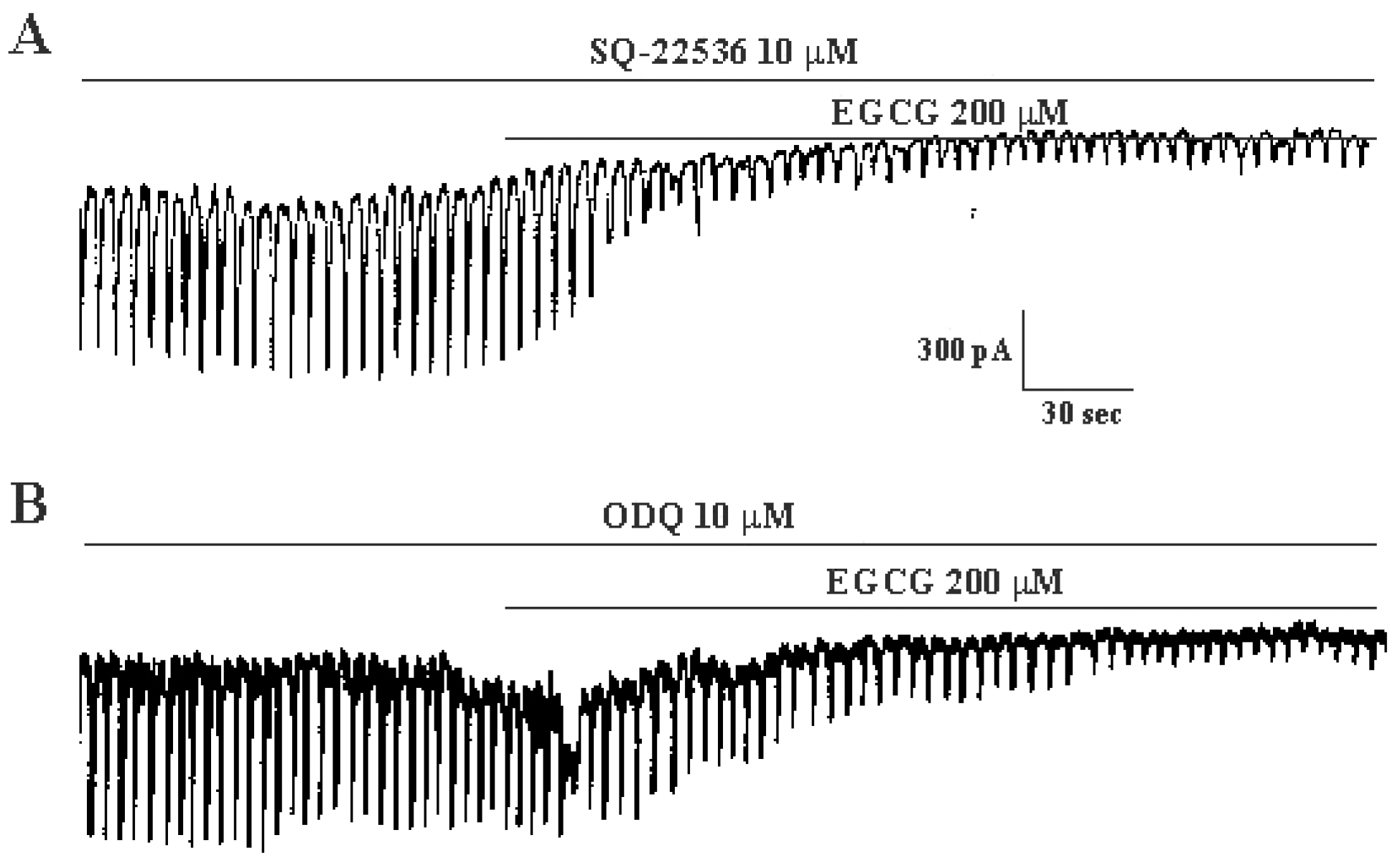
Fig. 4.
Effects of tetraethylammonium chloride, a Ca2+-activated K+ channel blocker, and glibenclamide, an ATP-sensitive K+ channel blocker, on (–)-epigallocatechin gallate-induced response in cultured ICC from mouse small intestine. (A) Pretreatment with tetraethylammonium chloride (2 mM) did not affect the inhibitory effects of (–)-epigallocatechin gallate (200 μM) on spontaneous inward currents. (B) Pretreatment with glibenclamide (10 μM) did not affect the inhibitory effects of (–)-epigallocatechin gallate (200 μM) on spontaneous inward currents. Vertical solid line scales amplitude of pacemaker current and horizontal solid line scales duration of recording (s) pacemaker currents. EGCG: (–)-epigallocatechin gallate, TEA: tetraethylammonium chloride.
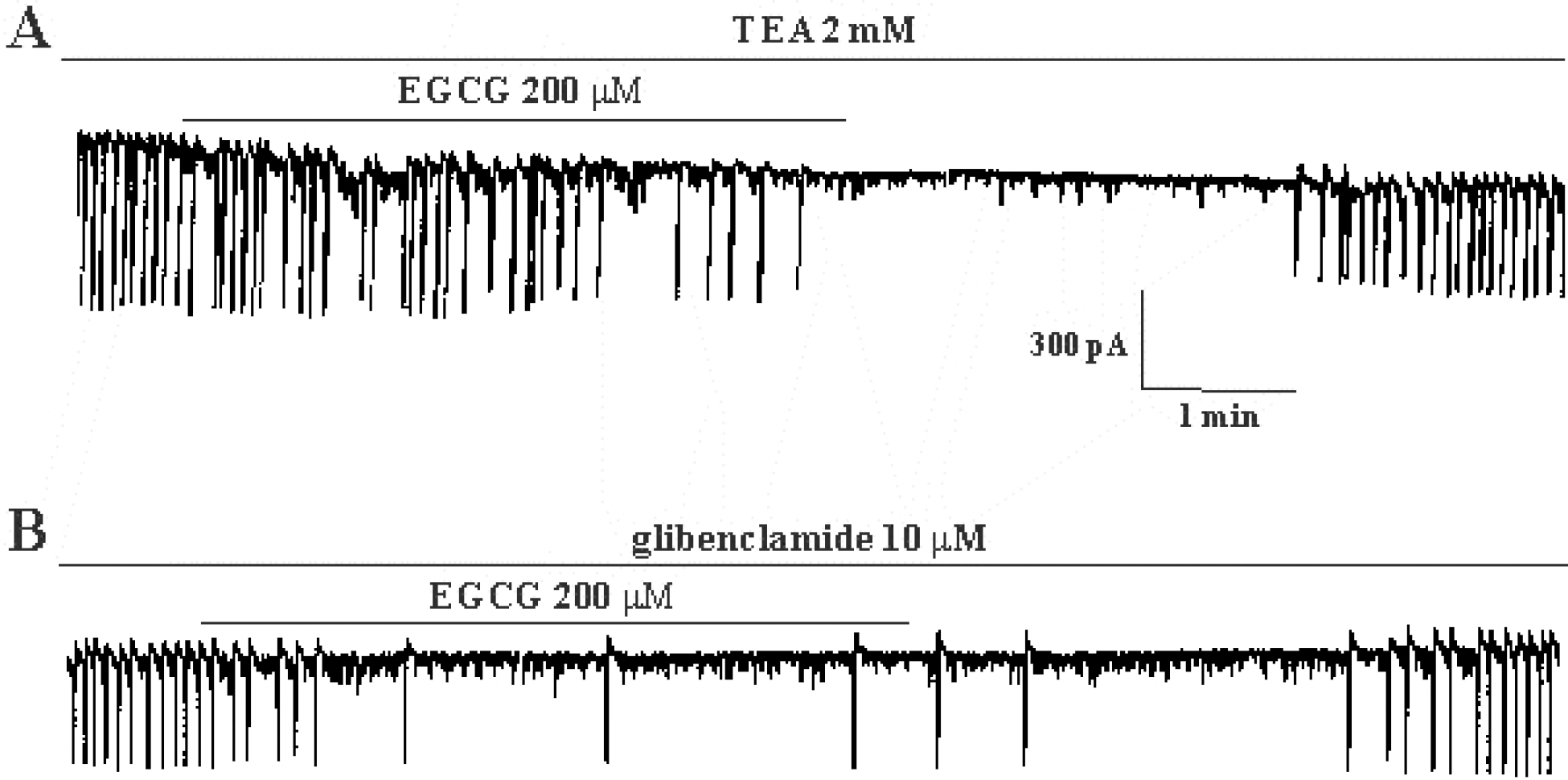
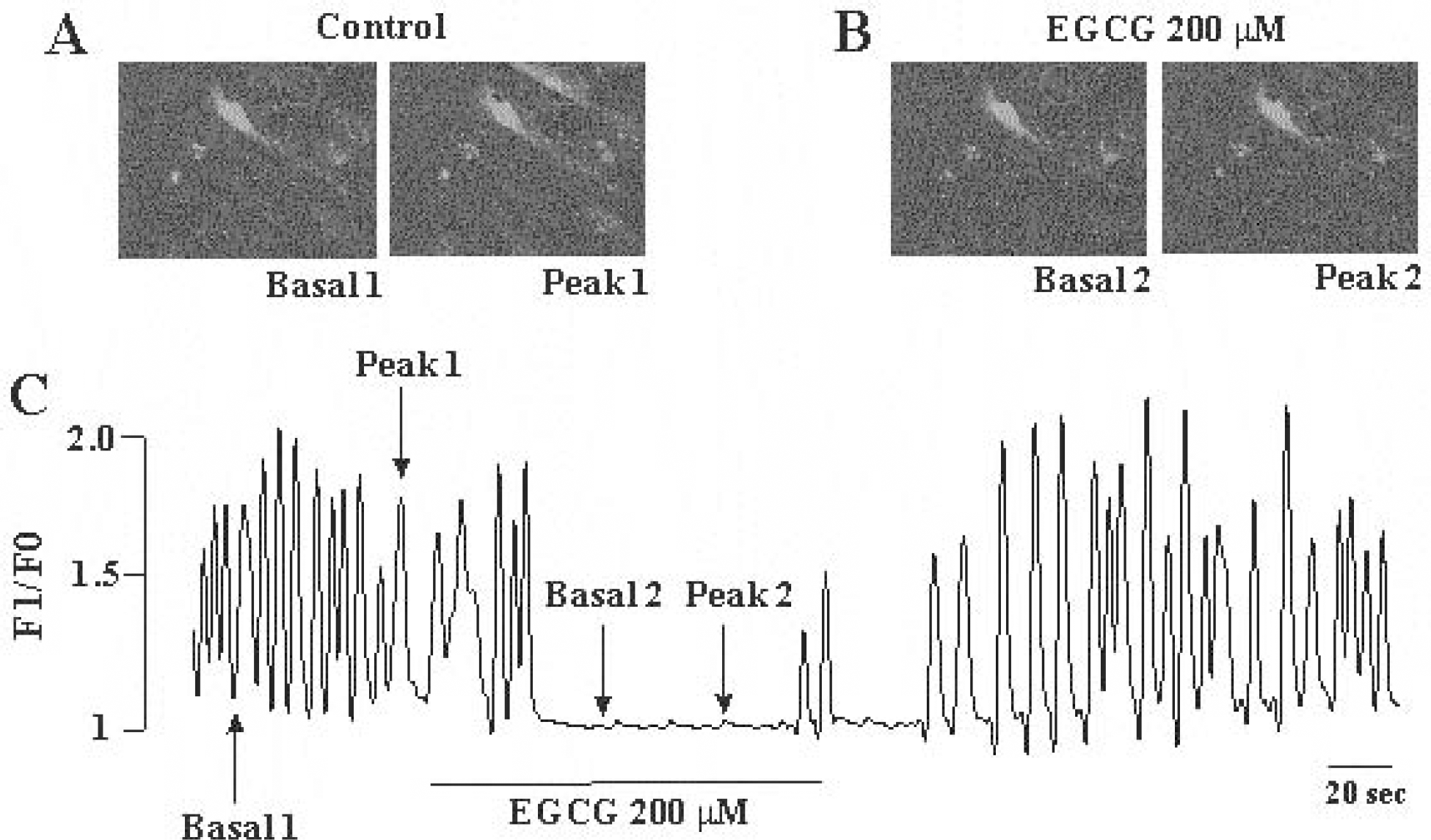
Fig. 5.
Effects of (–)-epigallocatechin gallate on intrcellular Ca2+ oscillation in cultured ICC from mouse small intestine. (A) Sequential fluorescence intensity images of fluo-3-loaded cultured ICC in control condition. (B) Sequential fluorescence intensity images of fluo-3-loaded cultured ICC in the presence of (–)-epigallocatechin gallate (200 μM). The images of basal (1 and 2) and peak (1 and 2) in (A) and (B) acquired from indicators in (B). (C) Fluorescence intensity change plotted in (A) and (B) red marker.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download