Abstract
Purpose
To investigate effects of a new push-through insertion method for donor lenticules using an injector system on endothelial viability ex vivo and in a clinical case series of endothelial keratoplasty.
Methods
An ex vivo delivery model was used with porcine corneoscleral rims. We compared the endothelial viability in a new push-through insertion method using the Visian Implantable Collamer Lens (ICL) injector versus that of standard forceps-assisted insertion for lenticule delivery. Twenty porcine corneal lenticules were divided into four groups by insertion method and wound size. Vital dye staining was performed and devitalized areas were semi-quantitatively assessed by digital imaging. In the clinical case series, Descemet's stripping endothelial keratoplasty (DSEK) using the push-through method was performed in seven patients and endothelial outcome was determined six months postoperatively.
Results
Mean devitalized areas for the push-through method were significantly lower than for forceps-assisted insertion through 3.2 mm incision (23.99 ± 2.17% vs. 50.48 ± 5.07%, p = 0.009) in the ex vivo model. Average endothelial cell counts of donor tissues of patients who underwent DSEK were 26.4% lower six months postoperatively.
Deep lamellar endothelial keratoplasty (DLEK) and Descemet's stripping endothelial keratoplasty (DSEK) were recently introduced, and have partly replaced full-thickness penetrating keratoplasty (PKP) during the early stage of bullous keratopathy [1]. The advantages of DLEK and DSEK over PKP include an intact anterior chamber during surgery, minimal post-surgical astigmatism, and more rapid visual rehabilitation [1-3].
However, the reduction of serious endothelial damage during donor delivery through the incision to the anterior chamber remains challenging because of the excessive amount of tissue manipulation of the donor lenticule during delivery. Furthermore, the current conventional insertion method of a "taco-shaped" folded donor lenticule through a small wound using a forceps [4,5] may cause unintended mechanical injury and resultant endothelial damage [6]. To minimize endothelial damage of the donor lenticule during delivery, various methods have been introduced, namely, folding of the donor lenticule with glides and cartridges [7-9], and intracameral tissue placement by pulling with either peripheral traction sutures or with a cross-chamber forceps [9-13]. Moreover, some advanced insertion devices have been introduced and tend to cause less traumatic tissue delivery, simultaneous maintenance of anterior chamber volume, and smaller incisions than are currently used [14,15]. However, most of these devices have not been approved by the US Food and Drug Administration (FDA).
One of the approaches involves pull-through delivery using a 23-gauge needle cystitome and a Visian Implantable Collamer Lens (ICL; STAAR Surgical, Monrovia, CA, USA) cartridge [8]. In this previous study, it was suggested that this method causes less endothelial cell damage than forceps insertion, because it allows the donor lenticule to be rolled into a compact shape while it travels through the cartridge, which avoids the compressive forces associated with folding. The main drawback of this procedure is that the needle can damage the edge of the donor lenticule and surrounding tissues when the needle is used to pull the donor lenticule across the anterior chamber. We adopted the use of an ICL cartridge to remove forceps-induced compression damage, as was done in this previous report, but we modified the insertion technique and used a sponge plunger to avoid needle-associated damage.
Therefore, we introduce a new push-through insertion method using the Visian ICL injector system for donor lenticule delivery. The method is easily performed, requires only a small incision, and does not require a needle. One of the main advantages of the push-through insertion method avoids full compression of the graft by obviating the use of a forceps. We also addressed the effect on endothelium of the push-through technique using the Visian ICL injector system for graft delivery. We discovered ex vivo that the push-through insertion method produced less endothelial damage than forceps-assisted delivery. A clinical case series also showed clinically acceptable endothelial cell loss six months after DSEK.
Twenty porcine eyeballs were prepared in an artificial chamber (Katena Products, Denville, NJ, USA) to produce donor lenticules. A 750 µm thick, 8.5 mm diameter anterior lamellar was trephined using a Barron recipient vacuum trephine (Katena Products), removed after manual dissection using a beaver blade (BD Ophthalmic Systems, Waltham, MA, USA). The thickness of the donor lenticules was aimed to be about 100 to 120 µm. Each corneal donor button was then placed endothelial side up on a Teflon corneal cutting block and punched out with an 8.0 mm diameter Barron donor vacuum punch (Katena Products). An ex vivo delivery model was prepared as previously described [16]. A 3.2 or 4.0 mm limbal incision was then made using a keratome. The 20 porcine donor lenticules were divided into four groups based on insertion method and wound size. Each group included five donor lenticule-delivered eyes. In the control group, donor lenticules were simply trephined without delivery. The injector group lenticules were delivered using the Visian ICL injector system through a 3.2 mm limbal incision. The forceps-assisted group (3.2 mm incision) lenticules were folded with a Kelman-Mcpherson corneal forceps (Rumex International, St. Petersburg, FL, USA) and transferred through a 3.2 mm limbal incision. The forceps-assisted group (4.0 mm incision) lenticules were folded with a Kelman-McPherson corneal forceps and carried through a 4.0 mm limbal incision.
In groups 3 and 4, lenticules were folded using a Kelman-Mcpherson corneal forceps into a taco shape, as previously described [16]. In group 2, endothelial-side up lenticules were carefully placed in a balanced salt solution (BSS)-filled cartridge in the shape of a half-rolled taco, endothelial side inside. After mounting the cartridge into the injector, lenticules were pushed forward using a sponge plunger and advanced through the cartridge into the anterior chamber. During the advance, we gently pushed the lenticules in order not to fold them. During all of the above steps, no ophthalmic viscosurgical device (OVD) was used in any group.
Trypan blue 0.25% (MP Biomedicals, Solon, OH, USA) was added drop-wise to cover the endothelium. After 120 seconds, the stain was removed and the donor lenticule was briefly rinsed twice in BSS, drained to remove excess saline, and placed on a glass slide endothelial surface up. The endothelial layer was then covered with alizarin red S 0.2% (GFS Chemicals, Columbus, OH, USA) for 90 seconds, the staining reagent was then removed, and samples were rinsed twice with BSS.
Lenticules were placed in a clear dish containing BSS and digital photographs were taken at the same magnifying power (×16). Computer digitized planimetry in Photoshop ver. 9.0 (Adobe Systems, San Jose, CA, USA) was used to semi-quantitatively assess devitalized areas of endothelium as previously described [17]. Briefly, we initially counted the number of pixels that composed stained damaged areas, and divided this number by the number of pixels that composed the entire endothelial area. Results were expressed as percent endothelial damage (Fig. 1).
Surgery was performed in seven eyes of seven patients by one surgeon (ESC) using the push-through technique and the Visian ICL injector system. Informed consent was obtained from all patients in accordance with institutional review board/ethics committee and followed the tenets of the Declaration of Helsinki.
Donor lenticules, mean diameter 7.5 mm and average thickness 166 mm, were prepared. Anterior lamellar tissue was removed using an automated microkeratome system (Moria ALTK System, Antony, France) in an artificial chamber (Bausch and Lomb, St. Louis, MO, USA). Endothelial lamellar buttons were then punched out using a Barron donor vacuum punch (Katena Products).
After creating a 3.2 mm corneal incision at the 12 o'clock position, Descemet's membrane containing endothelial cell layers was stripped using a reverse Sinskey hook (Bausch and Lomb). The lenticules were loaded in the cartridge in the shape of a half-rolled taco, endothelial layer inside. The lenticule was not folded, but rolled in the cartridge. With the chamber maintained by an infusion cannula, the ICL cartridge was launched into the anterior chamber, and the donor lenticule was then gently pushed in the anterior chamber using the sponge plunger. After the lenticule had been transferred into the anterior chamber, the cartridge was retracted. By injecting air through the paracentesis, the graft was slowly unfolded and centered. All wounds were secured with 10-0 nylon (Alcon, Fort Worth, TX, USA). Two-thirds of the anterior chamber was filled with air tamponade to ensure adhesion of the donor onto the posterior stromal surface of the recipient cornea (Fig. 2). Postoperatively, patients received topical antibiotics four times daily for the first 10 days. This was followed by topical prednisolone acetate (1%) six times daily for one week and then four times daily for the next three weeks. At one month postoperatively, the dose of topical prednisolone acetate (1%) was gradually tapered over six months and finally discontinued.
Preoperative donor endothelial cell densities were measured by specular microscopy in an Eye Bank Association of America-certified eye bank (Lions Eye Bank of Oregon, Portland, OR, USA). Postoperative endothelial cell densities were determined by confocal microscopy (ConfoScan4; Nidek Technologies, Padova, Italy) at three and six months postoperatively.
All data were analyzed using SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA). Analysis of variance was performed using the Kruskal-Wallis test and the Mann-Whitney U-test to compare differences between the various surgical techniques in the ex vivo model. Statistical significance was accepted for p-values of <0.05.
Fig. 3 presents average endothelial cell damage percentages after the insertion of a donor lenticule for each of the four groups. Fig. 4 shows vital dye stained and digitally analyzed corneal images in Adobe Photoshop. Surprisingly, the injector group displayed significantly less endothelial damage than forceps-assisted (3.2 mm, p = 0.009). In addition, the injector group also tended to demonstrate less endothelial cell damage than forceps-assisted group (4.0 mm), but this was not significant (p = 0.117). Mean endothelial cell devitalized areas in each group were 12.18 ± 2.02% (control), 23.99 ± 2.17% (injector group), 50.48 ± 5.07% (forceps-assisted group, 3.2 mm), and 31.44 ± 7.33% (forceps-assisted group, 4.0 mm), respectively (Fig. 3).
All patients achieved a visual acuity improvement after DSEK (Fig. 5). Mean time to complete resolution of corneal edema was 22.0 ± 10.4 weeks. No postoperative graft dislocations or other complications, such as glaucoma or rejection, occurred during the first six postoperative months. Mean preoperative endothelial cell density (ECD) was 2,522 ± 334 cells/mm2. Mean postoperative ECD was 2,046 ± 374 and 1,856 ± 416 cells/mm2 at three and six months postoperatively, respectively. Mean ECD was significantly decreased at three months postoperatively (p = 0.002), and stabilized at six months (Fig. 6). This decrease represented a 26.4% decline in donor endothelial cell count six months postoperatively, which seemed to be acceptable as compared with another report on the use of an injector during endothelial keratoplasty as considered in incision size [8]. Furthermore, this damage was less when compared with that reported for forceps use during endothelial keratoplasty [4-6], and with that reported for PKP [18,19].
This study shows that ex vivo push-through delivery produced less endothelial damage than forceps-assisted delivery, and our clinical cases suggest that the losses observed were clinically acceptable. These findings suggest that push-through delivery using an ICL injector represents an alternative to forceps-assisted delivery during endothelial keratoplasty.
The delivery of a donor lenticule during endothelial keratoplasty remains a difficult, demanding procedure. Since forceps-assisted folding and transfer was first introduced [4,5], a variety of methods have been devised to overcome compression damage by forceps [8,12,16,20-23]. Pulling the donor lenticule using forceps or needling at the opposite site across the anterior chamber seemed to do harm on the pulling margin. Pulling the lenticles may also damage the crystalline lens when the forceps or sutures pass across the lens. Most importantly, a lenticule could be contaminated or compressed when it would be passed through the incision. The recently devised catheter-based technologies mentioned above allow atraumatic tissue delivery [14,15]. However, most of these injectors have not been approved yet by the US FDA. However, the Visian ICL cartridge-based injecting device has been approved for use in ophthalmic surgery by the US FDA, and provides an option for other applications like endothelial keratoplasty. Push-through insertion using the Visian ICL injector could offer benefits similar to those provided by other injector-assisted delivery systems, including a smaller incision, less astigmatism, faster visual recovery, better anterior chamber stability, less manipulation of the rolled-graft (as compared with the folded-graft), and better stabilization of the anterior chamber due to the smaller incision used.
Meanwhile, our ex vivo model showed higher endothelial loss than other injectors. The reason seemed to be that the smaller diameter of the cartridge in the ICL injector may compress the lenticles to some extent. In fact, opposite margins of the lenticule seemed to be partially overlapped inside the cartridge although the graft was rolled.
In this study, we did not use OVDs during donor lenticule insertion ex vivo, because the use of an OVD might have disturbed vital staining and lead to an underestimate of real damage to endothelium. This is why endothelial cell damage was greater for donor lenticules delivered by forceps or an injector than non-delivered controls. No use of OVDs might also have contributed to the greater endothelial damage observed in the present study than in previous reports on other injectors [8,24,25]. Nevertheless, our data show that endothelium damage was less for injector-assisted delivery than conventional forceps-assisted delivery, which provides scientific evidence that is useful for clinicians.
In our interventional clinical series, the OVD was also minimally placed on the donor lenticule or cartridge. We were concerned that the OVD coating might disrupt adhesion between the graft and the posterior stromal surface or that excessive manipulation to remove OVDs could lead to graft dislodgement. The fact that OVDs were hardly used could be why we did not experience any displacement of grafts nor any increase in intraocular pressure. Although the present study should be considered a preliminary study due to the small number of cases enrolled and the relatively short-term follow-up, the clinically favorable outcomes achieved suggest that the push-through insertion method is a good option for endothelial keratoplasty.
In summary, we have devised a new means of delivering a donor lenticule that utilizes push-through insertion and the Visian ICL injector system. This technique might avoid folding of the donor lenticule and can be done via a small incision. It is straightforward and has a short learning curve. In addition, this delivery was found to be less harmful to donor endothelium than forceps delivery. These findings suggest that the push-through insertion method is a promising delivery option during endothelial keratoplasty.
Figures and Tables
Fig. 1
Computer digitized planimetry using Photoshop ver. 9.0 was used to semi-quantitatively assess devitalized areas of endothelium. To determine damaged endothelial area percentages, we used pixel counts. The red box in the picture displays the pixel count in devitalized areas (gray).
Fig. 2
The push-through insertion method using the Visian Implantable Collamer Lens (ICL) injector. (A) The donor lenticule was placed on the balanced salt solution filled ICL cartridge, (B) and then carefully advanced through the cartridge using the sponge plunger. (C) The donor lenticule was rolled not folded. (D) The photograph shows a cartridge mounted in the injector. (E) Photograph showing the injector inserted into the anterior chamber. (F) Photograph showing the donor lenticule being pushed into the anterior chamber. (G) Photograph showing unrolling of the donor lenticule as the injector is retracted. (H,I) Centration of the donor lenticule was performed through a venting stab incision.
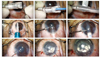
Fig. 3
Average endothelial cell damage percentages after the insertion of a donor lenticule in the four study groups. Group 1, control group (no delivery); group 2, Implantable Collamer Lens injector system-assisted delivery through a 3.2 mm limbal incision; group 3, forcep-assisted delivery through a 3.2 mm limbal incision; group 4, forcep-assisted delivery through a 4.0 mm limbal incision. Error bars: 95% confidence interval.

Fig. 4
Vital dye-stained corneal images and images analyzed using Adobe Photoshop. Group 1, control group (no delivery); group 2, Implantable Collamer Lens injector system-assisted delivery through a 3.2 mm limbal incision; group 3, forcep-assisted delivery through a 3.2 mm limbal incision; group 4, forcep-assisted delivery through a 4.0 mm limbal incision.

References
1. Price FW Jr, Price MO. Descemet's stripping with endothelial keratoplasty in 50 eyes: a refractive neutral corneal transplant. J Refract Surg. 2005. 21:339–345.
2. Chen ES, Terry MA, Shamie N, et al. Descemet-stripping automated endothelial keratoplasty: six-month results in a prospective study of 100 eyes. Cornea. 2008. 27:514–520.
3. Gorovoy MS. Descemet-stripping automated endothelial keratoplasty. Cornea. 2006. 25:886–889.
4. Melles GR, Wijdh RH, Nieuwendaal CP. A technique to excise the descemet membrane from a recipient cornea (descemetorhexis). Cornea. 2004. 23:286–288.
5. Melles GR. Posterior lamellar keratoplasty: DLEK to DSEK to DMEK. Cornea. 2006. 25:879–881.
6. Terry MA, Wall JM, Hoar KL, Ousley PJ. A prospective study of endothelial cell loss during the 2 years after deep lamellar endothelial keratoplasty. Ophthalmology. 2007. 114:631–639.
7. Mehta JS, Por YM, Beuerman RW, Tan DT. Glide insertion technique for donor cornea lenticule during Descemet's stripping automated endothelial keratoplasty. J Cataract Refract Surg. 2007. 33:1846–1850.
8. Kuo AN, Harvey TM, Afshari NA. Novel delivery method to reduce endothelial injury in descemet stripping automated endothelial keratoplasty. Am J Ophthalmol. 2008. 145:91–96.
9. Busin M, Bhatt PR. Late detachment of donor graft after Descemet stripping automated endothelial keratoplasty. J Cataract Refract Surg. 2008. 34:159–160.
10. Vajpayee RB, Agarwal T, Jhanji V, Sharma N. Modification in descemet-stripping automated endothelial keratoplasty: "Hitch suture" technique. Cornea. 2006. 25:1060–1062.
11. Macsai MS, Kara-Jose AC. Suture technique for Descemet stripping and endothelial keratoplasty. Cornea. 2007. 26:1123–1126.
12. Van Cleynenbreugel H, Hillenaar T, Remeijer L. Graft insertion during Descemet-stripping automated endothelial keratoplasty: pulling the graft inward. J Cataract Refract Surg. 2008. 34:534–536.
13. Sarnicola V, Toro P. Descemet-stripping automated endothelial keratoplasty by using suture for donor insertion. Cornea. 2008. 27:825–829.
14. Bethke W. Injecting innovation into DSEK. Rev Ophthalmol. 2008. 15:1.
15. Ide T. Descemet's stripping automated endothelial keratoplasty injecting device. Expert Rev Ophthalmol. 2009. 4:5–9.
16. Ide T, Yoo SH, Goldman JM, et al. Descemet-stripping automated endothelial keratoplasty: effect of inserting forceps on DSAEK donor tissue viability by using an in vitro delivery model and vital dye assay. Cornea. 2007. 26:1079–1081.
17. Saad HA, Terry MA, Shamie N, et al. An easy and inexpensive method for quantitative analysis of endothelial damage by using vital dye staining and Adobe Photoshop software. Cornea. 2008. 27:818–824.
18. Terry MA. Deep lamellar endothelial keratoplasty (DLEK): pursuing the ideal goals of endothelial replacement. Eye (Lond). 2003. 17:982–988.
19. Bohringer D, Reinhard T, Spelsberg H, Sundmacher R. Influencing factors on chronic endothelial cell loss characterised in a homogeneous group of patients. Br J Ophthalmol. 2002. 86:35–38.
20. Aralikatti A, Dean S, Busin M, Shah S. Pull-through technique for graft insertion in DSAEK. J Cataract Refract Surg. 2008. 34:341.
21. Bradley JC, McCartney DL. Descemet's stripping automated endothelial keratoplasty in intraoperative floppy-iris syndrome: suture-drag technique. J Cataract Refract Surg. 2007. 33:1149–1150.
22. Macaluso C. Closed-chamber pulling-injection system for donor graft insertion in endothelial keratoplasty. J Cataract Refract Surg. 2008. 34:353–356.
23. Busin M, Bhatt PR, Scorcia V. A modified technique for descemet membrane stripping automated endothelial keratoplasty to minimize endothelial cell loss. Arch Ophthalmol. 2008. 126:1133–1137.
24. Mehta JS, Por YM, Poh R, et al. Comparison of donor insertion techniques for descemet stripping automated endothelial keratoplasty. Arch Ophthalmol. 2008. 126:1383–1388.
25. Terry MA, Saad HA, Shamie N, et al. Endothelial keratoplasty: the influence of insertion techniques and incision size on donor endothelial survival. Cornea. 2009. 28:24–31.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download