Abstract
Figures and Tables
Fig. 1
Effects of ATME on the cytotoxicity of HUVECs, MIAPaCa-2, HepG2, and CT-26 cells. HUVECs (A), MIAPaCa-2 (B), HepG2 (C), and CT-26 (D) cells were incubated with various concentrations (0.05, 0.1, 0.2, 0.5, and 1 mg/mL) of ATME. After 48 hr, cytotoxicity was quantified by an MTT assay. Each bar represents the average±SE of three independent experiments.

Fig. 2
ATME inhibits the VEGF-induced proliferation and migration of HUVECs. (A) HUVECs were pretreated for 30 min with various concentrations (5, 10, and 25 µg/mL) of ATME before exposure to VEGF (20 ng/mL). After 48 hr, the number of cells was determined by an MTT assay. (B, C) HUVECs were treated with VEGF (20 ng/mL) in the presence or absence of ATME (25 µg/mL). Chemotactic migration after incubation in Transwell plates for 4 hr. (B) Representative migrated cells were photographed. (C) Cells that migrated to the bottom of the filter were counted using optical microscopy. The in vitro angiogenesis assay was performed as described in the Materials and Methods. Data are expressed as the means+SE (n = 3). *P < 0.01 vs. control and †P < 0.01 vs. VEGF alone.
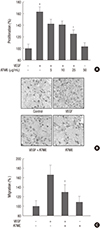
Fig. 3
ATME inhibits VEGF-induced invasion and tube formation of endothelial cells. (A) Effect of ATME on HUVEC invasion by using the Transwell culture plate. HUVECs were treated for 16 hr with VEGF (20 ng/mL) and various concentration (5, 10, 25, and 50 µg/mL) of ATME. VEGF treatment alone served as a positive control. (B, C) HUVECs were preincubated for 30 min with 25 µg/mL ATME and plated on Matrigel-coated plates at a density of 2×105 cells per well. They were incubated in the presence or absence of 20 ng/mL VEGF, and microphotographs were obtained after 20 hr (40×). (B) Representative endothelial tubes are shown. (C) The area covered by the tube network was measured using the Image-Pro Plus software. The experiments were repeated three times, and values are the means+SE values of triplicate determinations. *P < 0.01 vs. VEGF alone.

Fig. 4
ATME inhibits VEGF-induced vessel sprouting ex vivo. Aortae in Matrigel were exposed to VEGF (20 ng/mL) in the absence or presence of ATME (25 µg/mL) and stained with Diff-Quick on day 7. (A) Representative aortic rings were photographed. (B) ATME blocks VEGF-induced vessel sprouting. The assay was scored from 0 (least positive) to 5 (most positive), and the data are mean±SE values (n = 6). *P < 0.01 vs. VEGF alone.

Fig. 5
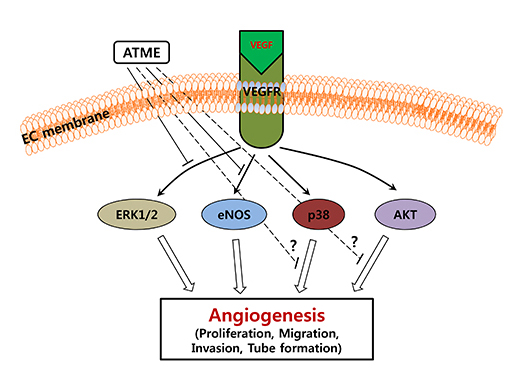
ATME inhibits VEGF-induced phosphorylation of p44/42 MAP kinase and eNOS but not phosphorylation of p38 and Akt. (A) HUVECs were pretreated for 30 min with various concentrations (5, 10, 25, and 50 µg/mL) of ATME and treated with VEGF (20 ng/mL) for 30 min. (B and C) HUVECs were incubated with 25 µg/mL ATME for 30 min and stimulated with VEGF (20 ng/mL) for the indicated times. At the indicated time points, cells were harvested and the levels of phosphorylated and total p44/42 MAP kinase, p38, Akt, and eNOS were determined by western blot analysis. Actin was used as a loading control.
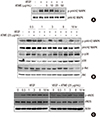
Fig. 6
Angiogenesis was reduced by ATME in the in vivo mouse Matrigel-plus assay. The experimental procedures are described under Materials and Methods. (A) The Matrigel without ATME did not show any inhibition of VEGF-induced migration or invasion of endothelial cells. However, with Matrigel containing ATME (25 µg), many blood vessels disappeared in the gel. (B) Quantitation of active vasculature inside the Matrigel by measurement of hemoglobin content. Each value represents the mean value for at least four animals, and similar results were obtained in three different experiments; bars, + SE. *P < 0.01 vs. VEGF alone.

ACKNOWLEDGEMENTS
Notes
This study is supported by a grant from Inha University and the Korea Healthcare technology R&D Project Ministry of Health and Welfare, Republic of Korea (HI14C1062).
AUTHOR CONTRIBUTION Conception and coordination of the study: Kim EC, Nam M. Design of ethical issues: Kim EC, Hong SS, Lee BI, Nam M. Acquisition of data: Kim EC, Kim SH, Piao SJ. Data review: Kim TJ, Bae K, Kim HS, Nam M. Statistical analysis: Kim EC, Kim SH, Piao SJ. Manuscript preparation: Kim EC, Piao SJ, Hong SS, Nam M. Manuscript approval: all authors.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download