Abstract
Figures and Tables
Fig. 2
Kaplan-Meier analysis for event free survival from total mortality, thromboembolic events, arrhythmic events and hospitalizations in both study groups.
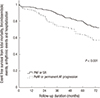
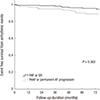
Fig. 4
Progression rates from PAF to PeAF or permanent AF. *Mean annual progression rate for 6 yr-10.7%/yr.

Table 1
Baseline clinical charactersistics in patients with PAF depending on the progression to persistent or permanent AF
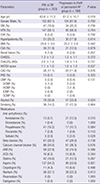
Values are mean±SD (range). PAF indicates paroxysmal atrial fibrillation; PeAF, persistent atrial fibrillation; AF, atrial fibrillation; SR, sinus rhythm; DM, diabetes mellitus; HTN, hypertension; CHF, congestive heart failure; BMI, body mass index; CVA, cerebrovascular accident; CHADS2 score, 1 point for congestive heart failure, hypertension, age [≥75 yr], diabetes mellitus, and 2 points for history of stroke or transient ischemic attack; CHA2DS2 VASc score, 1 point for congestive heart failure, hypertension, diabetes mellitus, vascular disease (previous myocardial infarction, complex aortic plaque, and peripheral artery disease [PAD]), age [65-74 yr] and 2 points for history of stroke or transient ischemic attack, age [≥75 yr]; HATCH score, 1 point for hypertension, age ≥75 yr, chronic obstructive pulmonary disease and 2 points for transient ischemic attack or stroke, heart failure (2 points); CAD, coronary artery disease; PAD, peripheral artery disease; CMP, cardiomyopathy; DCMP, dilated cardiomyopathy; HCMP, hypertrophic cardiomyopathy; ICMP, ischemic cardiomyopathy; SCMP, stress cardiomyopathy; ARB, angiotensin II receptor blocker; ACEi, angiotensin converting enzyme inhibitor.
Table 2
Baseline laboratory and echocardiographic findings in patients with PAF depending on the progression to persistent or permanent AF
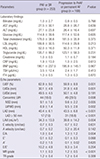
Values are mean±SD (range). PAF indicates paroxysmal atrial fibrillation; SR, sinus rhythm; PeAF, persistent atrial fibrillation; AF, atrial fibrillation; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDL, low density lipoprotein; HDL, high density lipoprotein; CRP, C-reactive protein; BNP, B type natriuretic peptide; TSH, thyroid stimulating hormone; fT4, free thyroxine; LVEF, left ventricular ejection fraction; LVIDd, left ventricular diastolic diameter; LVIDs, left ventricular systolic diameter; LVH, left ventricular hypertrophy; IVSD, interventricular septal diameter; LVPWD, left ventricular posterior wall diameter; LAD, left atrial diameter; LAVI, left atrial volume index; E, peak mitral flow velocity of the early rapid filling wave; A, peak velocity of the late filling wave due to atrial contraction; E', early diastolic mitral annular velocity; A', late diastolic mitral annular velocity; MR, mitral regurgitation; TR, tricuspid regurgitation.
Table 3
Clinical outcomes in patients with PAF at 6-year follow-up

Values are mean±SD (range). PAF indicates paroxysmal atrial fibrillation; SR, sinus rhythm; PeAF, persistent atrial fibrillation; AF, atrial fibrillation; PCI, percutaneous coronary intervention; CAG, coronary angiography; PPM, pacemaker insertion; CVA, cerebrovascular accidents; APC, atrial premature complex; ATach, atrial tachycardia; VPC, ventricular premature complex; RFCA, radiofrequency catheter ablation; LVEF, left ventricular ejection fraction; LVIDd, left ventricular diastolic diameter; LVIDs, left ventricular systolic diameter; LVH, left ventricular hypertrophy; IVSD, interventricular septal diameter; LVPWD, left ventricular posterior wall diameter; LAD, left atrial diameter; LAVI, left atrial volume index; E, peak mitral flow velocity of the early rapid filling wave; A, peak velocity of the late filling wave due to atrial contraction; E', early diastolic mitral annular velocity; A', late diastolic mitral annular velocity; MR, mitral regurgitation; TR, tricuspid regurgitation.
Table 4
Univariate and multivariate Cox analyses for progression from paroxysmal atrial fibrillation to persistent or permanent atrial fibrillation at 6-year follow-up

*Atrial arrhythmia indicates atrial arrhythmia during follow-up including atrial premature complex, atrial tachycardia and atrial flutter. OR, odds ratio; CI, confidence interval; BMI, body mass index; LVEF, left ventricular ejection fraction; LVH, left ventricular hypertrophy; LAD, left atrial diameter; MR, mitral regurgitation.
Notes
AUTHOR CONTRIBUTION Conception and coordination of the study: On YK, Im SI. Design of ethical issues: Park KM, On YK, Kim JS. Acquisition of data: Im SI, On YK. Data review: Im SI, On YK. Statistical analysis: Im SI, Park SJ, Huh J, On YK. Manuscript preparation: Im SI, On YK. Manuscript approval: all authors




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download