1. Giuliano AE, Dale PS, Turner RR, Morton DL, Evans SW, Krasne DL. Improved axillary staging of breast cancer with sentinel lymphadenectomy. Ann Surg. 1995; 222:394–399. discussion 9-401.
2. Na CJ, Kim J, Choi S, Han YH, Jeong HJ, Sohn MH, Youn HJ, Lim ST. The clinical value of hybrid sentinel lymphoscintigraphy to predict metastatic sentinel lymph nodes in breast cancer. Nucl Med Mol Imaging. 2015; 49:26–32.
3. Morton DL, Thompson JF, Essner R, Elashoff R, Stern SL, Nieweg OE, Roses DF, Karakousis CP, Mozzillo N, Reintgen D, et al. Validation of the accuracy of intraoperative lymphatic mapping and sentinel lymphadenectomy for early-stage melanoma: a multicenter trial. Multicenter Selective Lymphadenectomy Trial Group. Ann Surg. 1999; 230:453–463. discussion 63-5.
4. Nune SK, Gunda P, Majeti BK, Thallapally PK, Forrest ML. Advances in lymphatic imaging and drug delivery. Adv Drug Deliv Rev. 2011; 63:876–885.
5. Boolbol SK, Fey JV, Borgen PI, Heerdt AS, Montgomery LL, Paglia M, Petrek JA, Cody HS 3rd, Van Zee KJ. Intradermal isotope injection: a highly accurate method of lymphatic mapping in breast carcinoma. Ann Surg Oncol. 2001; 8:20–24.
6. Kim BT. Sentinel lymph node imaging in breast vancer. Korean J Nucl Med. 1999; 33:243–246.
7. Plut EM, Hinkle GH, Guo W, Lee RJ. Kit formulation for the preparation of radioactive blue liposomes for sentinel node lymphoscintigraphy. J Pharm Sci. 2002; 91:1717–1732.
8. Eshima D, Eshima LA, Gotti NM, Herda SC, Algozine CA, Burris TG, Vansant JP, Alazraki NP, Taylor AT. Technetium-99m-sulfur colloid for lymphoscintigraphy: effects of preparation parameters. J Nucl Med. 1996; 37:1575–1578.
9. Hung JC, Wiseman GA, Wahner HW, Mullan BP, Taggart TR, Dunn WL. Filtered technetium-99m-sulfur colloid evaluated for lymphoscintigraphy. J Nucl Med. 1995; 36:1895–1901.
10. Mariani G, Moresco L, Viale G, Villa G, Bagnasco M, Canavese G, Buscombe J, Strauss HW, Paganelli G. Radioguided sentinel lymph node biopsy in breast cancer surgery. J Nucl Med. 2001; 42:1198–1215.
11. Hauser W, Atkins HL, Richards P. Lymph node scanning with 99mTc-sulfur colloid. Radiology. 1969; 92:1369–1371.
12. Pedersen B, Kristensen K. Evaluation of methods for sizing of colloidal radiopharmaceuticals. Eur J Nucl Med. 1981; 6:521–526.
13. Tsopelas C. Particle size analysis of (99m)Tc-labeled and unlabeled antimony trisulfide and rhenium sulfide colloids intended for lymphoscintigraphic application. J Nucl Med. 2001; 42:460–466.
14. Tsopelas C. Lymphoscintigraphy is more effective using higher specific activity 99mTc -antimony trisulfide colloid in the rat. Hell J Nucl Med. 2014; 17:19–26.
15. Bombardieri E, Bonadonna G, Gianni L. Breast cancer: nuclear medicine in diagnosis and therapeutic options. Berlin: Springer;2007. p. 84.
16. Trifirò G, Viale G, Gentilini O, Travaini LL, Paganelli G. Sentinel node detection in pre-operative axillary staging. Eur J Nucl Med Mol Imaging. 2004; 31:S46–S55.
17. Cook SE, Park IK, Kim EM, Jeong HJ, Park TG, Choi YJ, Akaike T, Cho CS. Galactosylated polyethylenimine-graft-poly(vinyl pyrrolidone) as a hepatocyte-targeting gene carrier. J Control Release. 2005; 105:151–163.
18. Liu X, Xu Y, Wu Z, Chen H. Poly(N-vinylpyrrolidone)-modified surfaces for biomedical applications. Macromol Biosci. 2013; 13:147–154.
19. Wang L, Zeng R, Li C, Qiao R. Self-assembled polypeptide-block-poly (vinylpyrrolidone) as prospective drug-delivery systems. Colloids Surf B Biointerfaces. 2009; 74:284–292.
20. Saha GB. Fundamentals of nuclear pharmacy. New York: Springer;1997. p. 101.
21. Watanabe A. Cancer metastases research. 4th ed. New York: Nova Science Publishers;2008. p. 54–55.
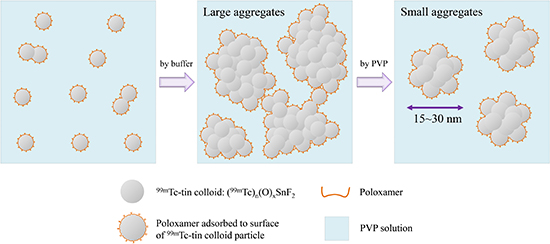
22. Illum L, Davis SS. The organ uptake of intravenously administered colloidal particles can be altered using a non-ionic surfactant (poloxamer 338). FEBS Lett. 1984; 167:79–82.
23. Higashi H, Natsugoe S, Uenosono Y, Ehi K, Arigami T, Nakabeppu Y, Nakajo M, Aikou T. Particle size of tin and phytate colloid in sentinel node identification. J Surg Res. 2004; 121:1–4.








 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download