Abstract
Figures and Tables
Fig. 1
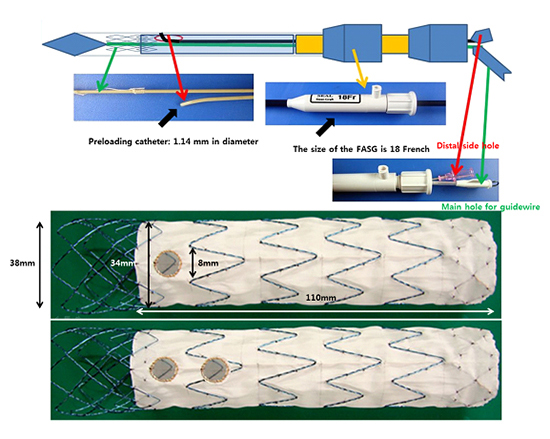
The fenestrated aortic stent graft (FASG) has a preloaded catheter (1.14 mm in diameter) inside the main delivery system for selection of the carotid artery, innominate artery, and subclavian artery (red arrow). The preloaded catheter links to a distal side hole (red arrow) 8 mm in diameter, and the distal port of the preloaded catheter protrudes from the distal portion of the deployment section of the main delivery system. A 0.035-inch guidewire can reach the carotid and subclavian arteries through the preloaded catheter. The size of the FASG is 18 French, including the preloaded catheter.

Fig. 2
The framework of the FASG is composed of 0.234-mm nitinol. The graft of the FASG is made of polytetrafluoroethylene. The FASG is 34 and 110 mm in diameter and length, respectively. The bare area of the FASG is 30 mm in length from the proximal end of the graft and oversized at 38 mm in diameter to prevent migration of the FASG. The proximal end of the FASG is tied up at 2 points to be movable when the FASG is deployed partially and when the FASG is deployed completely at the end of the stent graft deployment (A, B). The side hole is indicated with a circular gold mark, and either side of it is indicated with a straight gold mark to identify the side hole easily (C-E).

Fig. 3
Test of the deployment in vitro. We partially deployed the FASG up to the fenestration side holes (A) and then simulated the selection of carotid arteries using the guidewires. We then fully deployed the FASG (B) and finally untied the proximal end of the FASG (C, D).
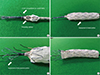
Fig. 4
The FASG was advanced into the aortic arch, and the fenestration side hole was kept facing the carotid artery. We then partially deployed the FASG up to the fenestration side holes (A, B) and selected carotid arteries with the 0.035-inch hydrophilic guidewires (C, D). We pushed the FASG up to fit the fenestration side holes into the carotid arteries (E) and deployed the FASG completely (F). The delivery sheath of the FASG was removed from the aorta, but the guidewires for the carotid arteries were maintained. We then advanced the stent grafts for the carotid arteries (G). Percutaneous transluminal balloons were then inflated to create good connections between the main FASG and stent grafts for the carotid arteries (H). Aortography was conducted to examine the flow of the carotid arteries and to detect endoleaks (I).

Fig. 6
Postmortem gross findings. There were no disconnections or tearing of the stent grafts, no fractures in the stent grafts, and no occlusion of the stent graft for the carotid arteries (A) for 1-branch (B) and 2-branch (C) FASG.
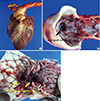
Table 1
Procedure-related findings in the one FASG (n = 6) and the two FASG group (n = 5)

ACKNOWLEDGEMENTS
Notes
This study was supported by Medical Research Institute Grant (2010-18), Pusan National University Hospital.
Conception and coordination of the study: Lee HC. Animal examination: Kim SP, Park TS, Ahn JH, Lee HW, Park JH, Lee HC. Acquisition of data: Lee HW, Oh J. Data review: Choi JH, Cha KW, Lee HC. Statistical analysis: Ahn KH, Lee HW. Manuscript preparation: Kim SP, Lee HC. Manuscript approval: all authors.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download