Abstract
Figures and Tables
Fig. 1
Flowchart of patient selection. (A) Patients with radical surgery. (B) Patients with additional surgeries after an endoscopic resection.
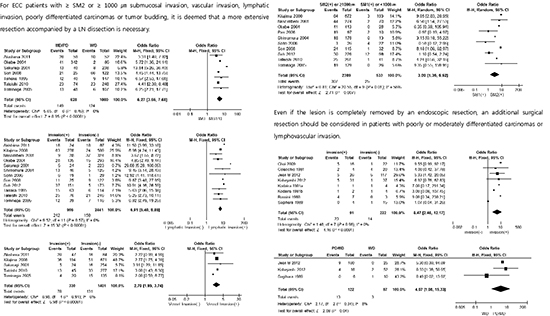
Fig. 2
Forest plots for lymph node metastasis in early colorectal carcinoma patients who underwent radical surgery. Categorized by (A) grossly depressed carcinoma vs. elevated carcinoma, (B) poorly or moderately differentiation vs. well differentiation, (C) lymphatic invasion vs. absence of lymphatic invasion, (D) vascular invasion vs. absence of vascular invasion. (E) SM2 or ≥ 1,000 µm vs. SM1 or < 1,000 µm, and (F) tumor budding vs. absence of tumor budding.

Fig. 3
Forest plots for lymph node metastasis in early colorectal carcinoma patients who underwent additional surgeries after an endoscopic resection. Categorized by (A) lymphovascular invasion vs. absence of lymphovascular invasion, (B) positive margin vs. clear margin at the time of endoscopic resection, and (C) poorly or moderately differentiation vs. well differentiation.

Table 1
Basic information for the included trials

| First author (yr) (Ref. No.) | Country | No. of patients | Type of therapy | Reason for additional surgery | LNM (%) | Analyzed risk factors of LNM |
|---|---|---|---|---|---|---|
| Akishima-Fukasawa Y (2011) (8) | Japan | 111 | Radical surgery | - | 32.4 | Lymphatic invasion |
| Kitajima K (2004) (9) | Japan | 865 | Radical surgery | - | 10.1 | Depth of invasion (SM depth ≥ 1,000 µm), Lymphatic invasion, Tumor budding |
| Nascimbeni R (2002) (10) | United States of America | 353 | Radical surgery | - | 13.0 | Depth of invasion (SM3), Lymphovascular invasion, Location (Lower rectum) |
| Okabe S (2004) (11) | United States of America, Japan | 428 | Radical surgery | - | 10.0 | Depth of invasion (SM depth > 3 mm), Lymphovascular invasion |
| Pan W (2006) (12) | Japan | 166 | Radical surgery | - | 6.6 | Depth of invasion, Lymphovascular invasion |
| Sakuragi M (2003) (13) | Japan | 278 | Radical surgery | - | 7.6 | Depth of invasion (SM depth ≥ 2,000 µm), Lymphatic invasion |
| Shimomura T (2004) (14) | Japan | 171 | Radical surgery | - | 10.5 | Depth of invasion (SM2, SM depth > 1,500 µm), Lymphatic invasion, Tumor budding |
| Sohn DK (2007) (15) | Korea | 48 | Radical surgery | - | 14.6 | Tumor budding |
| Son HJ (2008) (16) | Korea | 3,557 | Radical surgery | - | 17.0 | Depth of invasion (SM2 or SM3), Lymphatic invasion, Sex (Male), Location (Left side), Gross type (Depressed), Differentiation (Moderately or poorly differentiated carcinomas) |
| Suh JH (2012) (17) | Korea | 435 | Radical surgery | - | 13.0 | Lymphovascular invasion, Tumor budding, Differentiation (Undifferentiated carcinomas) |
| Tanaka S (1995) (4) | Japan | 177 | Radical surgery | - | 12.0 | Depth of invasion (SM depth > 400 µm), Lymphatic invasion, Gross type (Depressed), Differentiation (Undifferentiated carcinomas) |
| Tateishi Y (2010) (18) | Japan | 322 | Radical surgery | - | 14.3 | Lymphatic invasion, Differentiation (Undifferentiated carcinomas), Tumor budding |
| Tominaga K (2005) (19) | Japan | 155 | Radical surgery | - | 12.3 | Lymphatic invasion, Dedifferentiation (High-grade focal dedifferentiation) |
| Colacchio TA (1981) (20) | United States of America | 24 | Additional surgeries following polypectomy | Penetration of carcinoma into submucosa | 25.0 | - |
| Kodaira Sa (1981) (21) | Japan | 5 | Additional surgeries following polypectomy | Submucosally invasive carcinomas | 40.0 | - |
| Kodaira Sb (1981) (22) | Japan | 6 | Additional surgeries following polypectomy | Submucosally invasive carcinomas | 33.3 | - |
| Choi DH (2009) (23) | Korea | 38 | Additional surgeries following polypectomy | Poorly or undifferentiated carcinomas, Lymphovascular or venous invasion, Presence of tumor budding | 15.8 | Tumor budding |
| Rossini FP (1988) (24) | Italy | 10 | Additional surgeries following polypectomy | Poorly differentiated carcinomas, Lymphatic or vascular permeation, Resection margin involved with carcinoma | 40.0 | Lymphovascular invasion |
| Sugihara K32 (1989) (25) | Japan | 16 | Additional surgeries following polypectomy | Invasive carcinoma infiltrated within 1,000 µm from the edge, Venous invasion, Carcinoma infiltrating into more than 1/3 of the depth of the submucosa, Poorly differentiated adenocarcinoma | 6.3 | - |
| Butte JM (2012) (26) | United States of America | 143 | Additional surgeries following polypectomy | 6.9 | Young age, Lymphovascular invasion | |
| Kobayashi H (2012) (27) | Japan | 68 | Additional surgeries following polypectomy | Positive margin | 8.2 | Differentiation (Moderately or poorly differentiated carcinomas) |
| Lymphovascular invasion | ||||||
| Submucosally invasion (≥ 1,000 µm) |
Notes
Conception and design of the study: Jung SA, Cho WY. Acquisition of data: Choi JY, Cho WY. Analyzed the data: Kong KA. First draft of manuscript: Choi JY, Jung SA, Cho WY. Revision and critical review of the manuscript: Jung SA, Shim KN, Keum B, Byeon JS, Huh KC, Jang BI, Chang DK, Jung HY. ICMJE criteria for authorship read and met: Choi JY, Jung SA, Cho WY, Shim KN, Keum B, Byeon JS, Huh KC, Jang BI, Chang DK, Jung HY, Kong KA. Agree with manuscript results and conclusions: Choi JY, Jung SA, Cho WY, Shim KN, Keum B, Byeon JS, Huh KC, Jang BI, Chang DK, Jung HY, Kong KA.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download