Abstract
Several inflammatory markers have been investigated as prognostic parameters in a variety of cancer population with mostly favorable results. This study aimed to verify the significance of common inflammatory markers as prognostic variables and assess whether a selective combination of them as prognostic inflammation score (PIS) could further improve their prognostic values in surgical patients with colorectal cancer (CRC). A total of 265 patients who had undergone curative resection of CRC were reviewed retrospectively. Preoperative levels of inflammatory markers such as serum C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), white blood cell count (WBC), and neutrophil/lymphocyte ratio (NLR) were assessed by uni- and multivariate survival analysis with disease-free (DFS) and disease-specific survival (DSS). PIS was constructed with a selective combination of inflammatory markers which were independently significant. On univariate analysis, CRP, ESR, and NLR were significantly associated with DFS and DSS. On multivariate analysis, CRP and NLR were independently significant prognostic variables for DSS and DFS respectively (P=0.013, P=0.021). When PIS was constructed with combination of CRP and NLR, it was independently and significantly associated with both DFS and DSS (P=0.006, P=0.010). Furthermore, PIS was superior to CRP for DSS (HR=15.679 vs. HR=5.183), and NLR for DFS in terms of prognosticating power (HR=4.894 vs. HR=2.687). When PIS is constructed with combination of CRP and NLR, it is a potentially significant prognostic variable associated with poor survival regardless pathologic prognostic variables in patients with CRC after curative resection.
Graphical Abstract
Currently, the cornerstone in predicting cancer prognosis is accurate staging of the tumor with precise estimation of pathologic tumor spread, which is entirely tumor-related. However, there is increasing interest about patient-related prognostic factors such as systemic inflammatory response. There are several commonly used parameters for detecting systemic inflammation; serum level of C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), white blood cell count (WBC), and neutrophil/lymphocyte ratio (NLR). CRP is an essential acute phase reactant which is synthesized by hepatocytes in response to cytokine following inflammatory stimulus (1). ESR is the measurement of red blood cell aggregation which increases when an enhancement in the total mass exceeds the increase in volume. When an inflammatory process is present, the high proportion of fibrinogen in the blood causes red blood cells to stick to each other and increase their sedimentation (2). WBC is linked to neutrophilia which is a common host response to systemic inflammation. Because neutrophilia is often accompanied by lymphocytopenia which has also been suggested as a predictor of inflammation, NLR can be used as another index for the severity of systemic inflammation (34).
While many studies have been conducted to assess the prognostic significance of those inflammatory markers in a variety of cancer population including colorectal cancer (CRC), the measurement of systemic inflammation has been recently refined using a selective combination of each marker to improve the accuracy of predicting survival. Glasgow prognostic score with combination of CRP and albumin has been reported to have additional prognostic value in patients with various cancers (5). Prognostic index with combination of CRP and WBC has also been reported to be obviously associated with survival in patients with lung cancer (6).
This study aimed to verify the significance of commonly used inflammatory markers (CRP, ESR, WBC, and NLR) as prognostic variables and to assess whether a selective combination of them could further enhance their prognostic values as Prognostic inflammation score (PIS) in surgical patients with CRC.
All the patients who had undergone curative resection of CRC between January 2007 and October 2013 were reviewed, retrospectively. Among the patients, those with coexisting inflammatory conditions such as bowel perforation or obstruction, preoperative radiation or primary cancers in other organs, those died within 30 days of surgery and those without complete medical records or laboratory data were excluded. A total of 265 patients (163 males, 102 females) were included. Age was 67/34-95 (median/range) years. Follow-up time was 39/2-101 (median/range) months.
Database of the patients were searched to collect age (≤70, >70 yr), gender (male, female), tumor site (colon, rectum), preoperative laboratory results and postoperative pathologic results. Laboratory results included CRP, ESR, WBC, and NLR as inflammatory markers. Pathologic results included TNM stage of American Joint Committee on Cancer, cellular differentiation (well, moderate, and poor), lymphatic invasion (absent, present) and venous invasion (absent, present) as known prognostic variables.
Preoperative levels of inflammatory markers were determined with peripheral blood samples collected within 5 days before surgery. CRP was measured by latex-enhanced turbidimetric immunoassay, using a CRP latex (II) X2 (Denka Seiken Inc., Tokyo, Japan) with high-sensitivity application. Assay range of CRP was from 0.005 to 16 mg/dL. ESR was measured by TEST-1 SDL automated erythroid sediment rate analyzer (Alifax Inc., Polverara, Italy). WBC was determined using Sysmex XN-9000 hematology analyzer (Sysmex Inc., Hyogo, Japan). The NLR was calculated on the basis of WBC differential count with dividing the neutrophil count by the lymphocyte count.
Patients with stage III or IV colon cancer received postoperative chemotherapy. Some stage II patients who had pathological features of lymphatic/venous invasion or poor differentiation also received chemotherapy. Patients with stage II or III rectal cancer received postoperative chemoradiotherapy. Chemotherapy was done by standard regimens with 5-FU, oxaliplatin, or irinotecan. The patients receiving chemotherapy were required to have as Eastern Cooperative Oncology Group performance status of less than 3 and to have adequate hematopoietic, renal, and hepatic function. None of the patients had received postoperative therapy based on the preoperative data of inflammatory markers.
Patients were routinely followed up with three-month interval during first 3 yr postoperatively, six-month interval after three years, and annually after five years. When recurrence was detected, appropriate treatment including surgical resection of the lesion or palliative chemotherapy was done. Follow-up or survival time was defined as time from the date of surgery to last follow-up or death. Recurrence was defined as a lesion pathologically proven recurrent adenocarcinoma, or a lesion which was suspicious on imaging study including PET scan. Deaths caused by progression of the disease were considered as an end point for survival. Deaths due to other intercurrent condition were regarded as censored observations.
PIS was constructed with a selective combination of preoperative levels of inflammatory markers, which were independently significant in survival analysis. Score allocation was done according to the number of elevated markers. When only one marker was elevated above cut-off value, score 1 was given, and when two markers were elevated, score 2 was given. When no marker was elevated, score 0 was given.
The best discriminating cut-off values for inflammatory markers were defined by receiver-operating characteristic (ROC) curve analysis with disease-specific death as end-point. Relationships between inflammatory markers and clinicopathologic variables were assessed using contingency table with the chi-square test or log-likelihood ratio test. Survival analysis was done by Cox proportional hazards model (uni- and multivariate analysis, disease-free survival [DFS] and disease-specific survival [DSS]). Survival curves were made by Kaplan-Meier method and compared using log-rank test. A P value of less than 0.05 was considered significant. Statistical analysis was performed by dBSTAT 5.0 (dBSTAT Inc., Seoul, Korea) and SPSS 17.0 (SPSS Inc., Chicago, IL, USA).
Cut-off values for each inflammatory marker defined from ROC curve analysis with disease-specific death as end-point were 0.4 mg/dL for CRP, 15 mm/h for ESR, 7.3×109/L for WBC, and 2.4 for NLR. The areas under the curves were 0.745, 0.664, 0.605, and 0.678 in order of CRP, ESR, WBC, and NLR. Clinicopathologic characteristics of patients according to each inflammatory marker are shown in Table 1. There were no significant differences between the groups, except for age in CRP (P=0.004), ESR (P=0.012), and NLR groups (P=0.017), gender in ESR groups (P=0.020), and stage in CRP (P=0.001) and ESR groups (P<0.001).
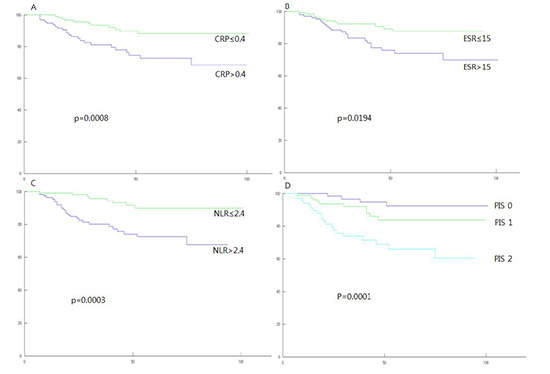
On univariate survival analysis, CRP, ESR, and NLR among inflammatory markers were significantly associated with both DFS and DSS. Stage, lymphatic invasion and venous invasion among clinicopathologic variables were also significantly associated with both DFS and DSS (Table 2). Cellular differentiation could not be evaluated due to biased distribution. On multivariate analysis performed including all above significant prognostic variables, CRP remained as independent and significant prognostic variable for DSS (HR=5.183, P=0.013), and NLR remained as such for DFS (HR=2.687, P=0.021) (Table 3). When PIS was constructed with combination of CRP and NLR, it was independently significant for both DFS and DSS (HR= 4.894, P=0.006 for DFS; HR=15.679, P=0.010 for DSS) (Table 4). Furthermore, it was superior to CRP for DSS (HR=15.679 vs. HR=5.183) and also superior to NLR for DFS (HR=4.894 vs. HR=2.687) in terms of prognosticating power (Tables 3, 4).
When stratified by PIS, 98 patients (37.0%) belonged to group of PIS 0, 89 patients (33.6%) to PIS 1, and 78 patients (29.4%) to PIS 2. Clinicopathologic characteristics of those patients are shown in Table 5. During the course of follow-up, 34 patients experienced recurrences. Thirty-three patients died, in whom 22 patients were due to progression of their cancers, 11 patients were due to other intercurrent diseases (Table 6). Stepwise association of PIS with DFS and DSS observed in survival curve analyses were shown in Fig. 1 and 2.
Inflammation has been known to have several ways of linkage with cancer development and progression. Chronic inflammation may cause not only excessive cell proliferation, but also activation of a cascade of cellular action, which can potentiate tumor cell growth. Besides, tumor growth itself can evoke more than normal host immune response and inflammation (7). With these backgrounds, the clinical use of readily available serum markers of systemic inflammation has been attempted to make an improvement in predicting cancer prognosis. Over the recent 10 yr, several inflammatory markers have been investigated whether they can be used for a prognostic parameter independent of TNM stage in a variety of cancer population including CRC with mostly favorable results (891011121314).
Recent studies indicated subclinical or even undetectable inflammation may also be as important as chronic inflammation in increasing cancer risk (15). Then the combination of multiple markers which can reflect various aspects of systemic inflammation is warranted for defining more meaningful prognostic parameters in patients with cancer. Actually the measurement of systemic inflammation has been subsequently refined with a selective combination of each marker. Glasgow prognostic score (5) with combination of CRP and albumin, Prognostic index (6) with CRP and WBC, and even Prognostic nutritional index (16) with albumin and lymphocyte have been reported to have additional prognostic values in various cancer populations. However, circulating albumin concentration is an indirect parameter of systemic inflammation. It reflects more of the nutritional status, rather than the severity of systemic inflammation, although it may be helpful for predicting survival of patients.
As laboratory markers reflecting systemic inflammation, CRP, ESR, WBC, and NLR have been widely used. However, it has been frequently noted that concomitant measurement of these markers gives discrepant results, which means that there would be differences among them theoretically or technically in measuring systemic inflammation (1718). CRP yields quick and sharp variations related to the extent and severity of the inflammation. False-negative or positive results are rare except in case of liver failure (19). CRP was confirmed again in this study to be independently significant prognostic variable in surgical patients with CRC. On the other hand, ESR is so slow in variation that it is subject to misinterpretation. Moreover, common associated conditions influencing its measurement may make it spurious (2021). These may be underlying causes of relatively poor significance of ESR as a prognostic variable in this study.
WBC is a simple and robust laboratory variable easily obtainable from routine blood test, and yet it is considered as a dynamic parameter which shows as sharp variation along with infection and inflammation. But its accuracy is more effective in acute, rather than chronic condition (3). Unlike in previous study which was done on patients with lung cancer (6), WBC did not show any significance by itself as a prognostic variable in this study. NLR is a parameter based on differential count of WBC. It reflects not only neutrophilia, but also lymphocytopenia. In response to systemic inflammation, WBC populations usually change with rapid kinetics, reflecting the role of neutrophils in the early stage of the inflammation. Neutrophilia is often accompanied by lymphocytopenia, which develops probably to suppress the adaptive immune response in favor of innate immunity (322). Oncologically neutrophilia may aid in the development and progression of cancer by providing an adequate environment for it to grow, and lymphocytopenia may also worsen the prognosis of cancer through the linkage to inadequate lymphocyte-mediated immune response to cancer (23). NLR was also confirmed in this study as an independently significant prognostic variable in surgical patients with CRC.
In this study, when PIS was constructed with combination of CRP and NLR, it was more potentially significant prognostic variable in surgical patients with CRC. CRP and NLR were chosen for combination because they were confirmed to be independently significant prognostic variables for DSS and DFS respectively. Although current cornerstone in predicting cancer prognosis is TNM staging, it is not amenable to assessment preoperatively. Because PIS can be assessed preoperatively, it would be used as a guide for deciding whether to perform palliative surgery or preoperative neoadjuvant therapy in patients with stage IV CRC. It could also be a supplemental guide for postoperative adjuvant therapy in patients with stage II CRC.
As a limitation of this study, number of patients included was relatively small and deceased patients were also proportionally small. It might reduce the statistical power. Sample size was reduced because a number of patients were excluded due to incomplete laboratory data. It might be inevitable considering the retrospective nature of this study. Further prospective study with a larger cohort of patients would be necessary to confirm the results of this study. As another limitation, cut-off values to categorize each group of inflammatory markers were defined by ROC curve analysis. They could be different from well-known normal range values, which the previous studies used frequently as their discriminating values. But I believe ROC curve analysis with disease-specific death as an end point is better in defining discriminating values for prognostic variable.
In conclusion, CRP and NLR among serum inflammatory markers were found to be independently significant prognostic variables for DSS and DFS, respectively. When PIS was constructed with combination of CRP and NLR, it was more potentially significant prognostic variable regardless of pathologic variables in surgical patients with CRC.
Figures and Tables
Fig. 1
Disease-free survival curves (Kaplan-Meier method with log-rank test). Vertical axis is survival rate (%), horizontal axis is follow-up time (months). (A) Groups categorized by C-reactive protein; (B) by erythrocyte sedimentation rate; (C) by neutrophil/lymphocyte ratio; (D) by Prognostic inflammation score.

Fig. 2
Disease-specific survival curves (Kaplan-Meier method with log-rank test). Vertical axis is survival rate (%), horizontal axis is follow-up time (months). (A) Groups categorized by C-reactive protein; (B) by erythrocyte sedimentation rate; (C) by neutrophil/lymphocyte ratio; (D) by Prognostic inflammation score.

Table 1
Clinicopathologic characteristics of patients according to each inflammatory marker

Table 2
Univariate analysis for prognostic variables

Table 3
Multivariate analysis for prognostic variables

Table 4
Multivariate analysis for PIS and other prognostic variables

Table 5
Clinicopathologic characteristics of patients according to PIS
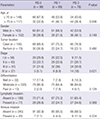
Data given in parenthesis is percentage in each group. PIS, prognostic inflammation score; PIS 0, C-reactive protein ≤0.4 mg/dL and neutrophil/lymphocyte ratio≤2.4; PIS 1, C-reactive protein >0.4 mg/dL or neutrophil/lymphocyte ratio>2.4; PIS 2, C-reactive protein >0.4 mg/dL and neutrophil/lymphocyte ratio>2.4.
References
1. Mazhar D, Ngan S. C-reactive protein and colorectal cancer. QJM. 2006; 99:555–559.
2. Sox HC Jr, Liang MH. The erythrocyte sedimentation rate. Guidelines for rational use. Ann Intern Med. 1986; 104:515–523.
3. Wyllie DH, Bowler IC, Peto TE. Relation between lymphopenia and bacteraemia in UK adults with medical emergencies. J Clin Pathol. 2004; 57:950–955.
4. Zahorec R. Ratio of neutrophil to lymphocyte counts: rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001; 102:5–14.
5. Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dunlop DJ. Comparison of an inflammation-based prognostic score (GPS) with performance status (ECOG) in patients receiving platinum-based chemotherapy for inoperable non-small-cell lung cancer. Br J Cancer. 2004; 90:1704–1706.
6. Kasymjanova G, MacDonald N, Agulnik JS, Cohen V, Pepe C, Kreisman H, Sharma R, Small D. The predictive value of pre-treatment inflammatory markers in advanced non-small-cell lung cancer. Curr Oncol. 2010; 17:52–58.
7. Siemes C, Visser LE, Coebergh JW, Splinter TA, Witteman JC, Uitterlinden AG, Hofman A, Pols HA, Stricker BH. C-reactive protein levels, variation in the C-reactive protein gene, and cancer risk: the Rotterdam Study. J Clin Oncol. 2006; 24:5216–5222.
8. McMillan DC, Canna K, McArdle CS. Systemic inflammatory response predicts survival following curative resection of colorectal cancer. Br J Surg. 2003; 90:215–219.
9. Nozoe T, Mori E, Takahashi I, Ezaki T. Preoperative elevation of serum C-reactive protein as an independent prognostic indicator of colorectal carcinoma. Surg Today. 2008; 38:597–602.
10. Sengupta S, Lohse CM, Cheville JC, Leibovich BC, Thompson RH, Webster WS, Frank I, Zincke H, Blute ML, Kwon ED. The preoperative erythrocyte sedimentation rate is an independent prognostic factor in renal cell carcinoma. Cancer. 2006; 106:304–312.
11. Choi ES, Kim HS, Han I. Elevated preoperative systemic inflammatory markers predict poor outcome in localized soft tissue sarcoma. Ann Surg Oncol. 2014; 21:778–785.
12. Teramukai S, Kitano T, Kishida Y, Kawahara M, Kubota K, Komuta K, Minato K, Mio T, Fujita Y, Yonei T, et al. Pretreatment neutrophil count as an independent prognostic factor in advanced non-small-cell lung cancer: an analysis of Japan Multinational Trial Organisation LC00-03. Eur J Cancer. 2009; 45:1950–1958.
13. Chua W, Charles KA, Baracos VE, Clarke SJ. Neutrophil/lymphocyte ratio predicts chemotherapy outcomes in patients with advanced colorectal cancer. Br J Cancer. 2011; 104:1288–1295.
14. Smith RA, Bosonnet L, Raraty M, Sutton R, Neoptolemos JP, Campbell F, Ghaneh P. Preoperative platelet-lymphocyte ratio is an independent significant prognostic marker in resected pancreatic ductal adenocarcinoma. Am J Surg. 2009; 197:466–472.
15. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010; 140:883–899.
16. Nozoe T, Kimura Y, Ishida M, Saeki H, Korenaga D, Sugimachi K. Correlation of pre-operative nutritional condition with post-operative complications in surgical treatment for oesophageal carcinoma. Eur J Surg Oncol. 2002; 28:396–400.
17. Costenbader KH, Chibnik LB, Schur PH. Discordance between erythrocyte sedimentation rate and C-reactive protein measurements: clinical significance. Clin Exp Rheumatol. 2007; 25:746–749.
18. Colombet I, Pouchot J, Kronz V, Hanras X, Capron L, Durieux P, Wyplosz B. Agreement between erythrocyte sedimentation rate and C-reactive protein in hospital practice. Am J Med. 2010; 123:863.e7–863.e13.
19. Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003; 111:1805–1812.
20. Jurado RL. Why shouldn't we determine the erythrocyte sedimentation rate? Clin Infect Dis. 2001; 33:548–549.
21. Hansson LO, Carlsson I, Hansson E, Hovelius B, Svensson P, Tryding N. Measurement of C-reactive protein and the erythrocyte sedimentation rate in general practice. Scand J Prim Health Care. 1995; 13:39–45.
22. Holub M, Beran O, Kaspříková N, Chalupa P. Neutrophil to lymphocyte count ratio as a biomarker of bacterial infections. Cent Eur J Med. 2012; 7:258–261.
23. Okano K, Maeba T, Moroguchi A, Ishimura K, Karasawa Y, Izuishi K, Goda F, Usuki H, Wakabayashi H, Maeta H. Lymphocytic infiltration surrounding liver metastases from colorectal cancer. J Surg Oncol. 2003; 82:28–33.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download