Abstract
Graphical Abstract
Figures and Tables
Fig. 1
Imaging of a 39-yr-old woman with a 37-mm-sized invasive ductal carcinoma in her left breast. (A) The first axial diffusion-weighted image (b value, 800 sec/mm2), showing a high-signal malignant mass in the left breast. (B) The region of interest (ROI) for the malignant mass was drawn manually (left), and the apparent diffusion coefficient (ADC) was calculated automatically in the ADC map. (C, D) Similarly, the ROI for normal tissue in the contralateral breast was drawn manually (right), and the ADC was calculated automatically. The same methods then were repeated. (E) The high-resolution postcontrast subtraction image was correlated.
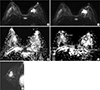
Fig. 2
Imaging of a 44-yr-old woman with a 26-mm-sized invasive ductal carcinoma in her left breast. (A) The first axial diffusion-weighted image (b value, 800 sec/mm2), showing a high-signal malignant mass in the left breast. (B) The region of interest (ROI) for the malignant mass was drawn manually (left), and the apparent diffusion coefficient (ADC) was calculated automatically in the ADC map. (C and D) Similarly, the ROI for normal tissue in the contralateral breast was drawn manually (right), and the ADC was calculated automatically. The same methods then were repeated. (E) The high-resolution postcontrast subtraction image was correlated.
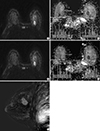
Fig. 3
Bland-Altman plots, showing the reproducibility of ADC measurements with repeated diffusion-weighted imaging (DWI) of malignant masses and normal breast tissue for reader 1 (A, B) and reader 2 (C, D). The x-axis shows the mean ADC measurements on repeated DWI, and the y-axis shows the difference between the ADC measurements of each set as a percentage of their mean. (blue thick solid line = mean absolute difference; red dashed line = 95% limits of agreement).

Fig. 4
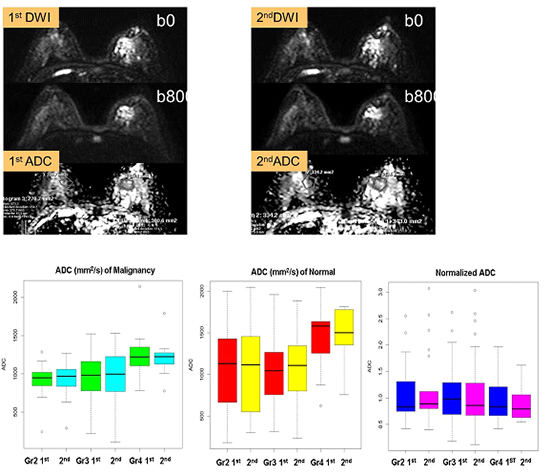
Graph, showing apparent diffusion coefficient (ADC) measurements based on mammographic density. The x-axis shows the mean ADC measurements on repeated diffusion-weighted imaging, according to the mammographic parenchymal density. The ADC measurements show relative differences in malignant breast masses with extremely dense parenchyma. Adjusted ADC values show consistency irrespective of mammographic density. 1st, first measured ADC; 2nd, second measured ADC; Gr, mammographic density grade.

Table 1
Imaging parameters for breast diffusion-weighted imaging

Table 2
Mean ADCs of 49 malignant breast masses vs. normal breast tissue

Table 3
Reproducibility of ADC measurement

Table 4
ICCs for ADC measurements based on mammographic density, lesion size, microcalcifications and DCIS component

Notes
Funding This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning(grant number 2014R1A1A1003355).
AUTHOR CONTRIBUTION Conception and design of the study: Jang M, Kim SM, Kang E, Kim SW. Acquisition of data: Jang M, Kim SM, Ahn HS, Yun BL. Statistical analysis: Jang M, Kim SY. First draft of the manuscript: Jang M. Revision and critical review of the manuscript and manuscript approval: all authors.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download