Abstract
Figures and Tables
Fig. 1
Dose-volume histograms (DVHs) of the normal liver (total liver volume minus planning target volume) from all patients. Grey color indicates patients with a CP score of 5; blue color with a CP score of 6; green color with a CP score of 7; red color with a CP score of 8. Solid lines represent patients without hepatic toxicity; dotted line with hepatic toxicity ≥grade 2.

Table 1
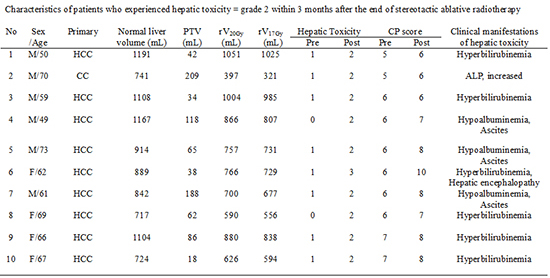
Characteristics of patients who experienced hepatic toxicity ≥ grade 2 within 3 months after the end of stereotactic ablative radiotherapy (SABR)

*Was scored according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v4.0; †was accompanied with diarrhea at 2 weeks after the end of SABR. HCC, hepatocellular carcinoma; CC, cholangiocarcinoma; Normal liver volume, total liver volume minus planning target volume (PTV); CP score, Child-Pugh score; ALP, alkaline phosphatase; rVxGy, normal liver volume receiving <X Gy, reverse VxGy.
Table 2
Univariate analysis for clinical predictors affecting hepatic toxicity ≥ grade 2 after stereotactic ablative radiotherapy (SABR)
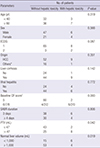
Table 3
Univariate analysis for dosimetric predictors affecting hepatic toxicity ≥ grade 2 after stereotactic ablative radiotherapy
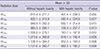
Table 4
Hepatic toxicity from prospective studies using stereotactic ablative radiotherapy for primary and metastatic liver cancers, which had a good baseline liver function and satisfied their own liver dose constraints in all patients

| Study design/Author | Origin (No. of pts) | CP class | Median dose (range, Gy)/ fx's | Liver dose constraints | Incidence of hepatic toxicity |
|---|---|---|---|---|---|
| Phase I | HCC (2) | *1 | 51(36-60)/3 | rV15Gy ≥ 700 mL | No |
| Schefter (26) | Liver mets (16) | ||||
| Phase I/II | Liver mets (36) | †2 | 60 (36-60)/3 | rV15Gy ≥ 700 mL | No |
| Kavanagh (27) | |||||
| Phase I/II | Liver mets (47) | *1 | 60 (36-60)/3 | rV15Gy ≥ 700 mL | No |
| Rusthoven (28) | |||||
| Phase I | Liver mets (27) | A | 50 (30,50,60)/5(3-5) | rV15Gy ≥ 700 mL in 3 fx's | No |
| Rule (29) | rV21Gy ≥ 700 mL in 5 fx's |
*1adequate liver function was defined as total bilirubin <3 mg/dL, serum albumin >2.5 g/dL, serum levels of liver enzymes <3 times the upper limit of normal, and normal prothrombin time (PT) and partial thromboplastin time (PTT) unless the patient was receiving anticoagulant medication; †2adequate liver function was defined as total bilirubin <3 mg/dL, albumin >2.5 g/dL, and normal PT/PTT unless on anticoagulants. CP class, Child-Pugh class; HCC, hepatocellular carcinoma; Liver mets, liver metastases; rVxGy, normal liver volume receiving <X Gy, reverse VxGy; RILD, radiation-induced liver disease.
Table 5
Hepatic toxicity from prospective or retrospective studies using stereotactic ablative radiotherapy for primary and metastatic liver cancers, which had patients with a Child-Pugh (CP) class of B and did not strictly satisfy their own liver dose constraints in all patients

| Study design/Author | Origin (No. of pts) | CP class | Median dose (range, Gy)/ fx's | Liver dose constraints | Incidence of hepatic toxicity | Predictor for hepatic toxicity |
|---|---|---|---|---|---|---|
| Phase I | HCC (17) | A, B | 36-48/3 in CP-A | rV17Gy ≥ 700 mL | Grade 3:12% | CP score |
| Cardenes (8) | 40/5 in CP-B | |||||
| Retrospective | HCC (36) | A, B, C | 36 (30-39)/3 | V20Gy < 50% | ≥ Grade 2: 33% | V10Gy for ≥ grade 2 V18Gy for CP class progression |
| Son (9) | CP class progression: 11% | |||||
| Phase I/II | HCC (60) | A, B | 44 (30-48)/3 in CP-A | CP-A: D33% ≤ 10 Gy | ↑ in hematologic/hepatic toxicity ≥ 1 grade: 13% | CP score for toxicity ≥ 1 grade |
| Andolino (10) | 40 (24-48)/5 in CP-B | +rV7Gy ≥ 500 mL | ||||
| CP-B: D33% ≤ 18 Gy | CP class progression: 20% | |||||
| +rV12Gy ≥ 500 mL | ||||||
| Retrospective | HCC (75) | A, B | 45 (24-45)/3 | rV17Gy > 700 mL | Ascites: 7% | Normal liver volume |
| Bibault (11) | +V15Gy < 50% | |||||
| +V21Gy < 33% | ||||||
| Retrospective | HCC (92) | A, B | 45 (30-60)/3-4 | NS | ≥ Grade 2: 19% | CP class |
| Jung (12) | ||||||
| Retrospective | HCC (21) | A, B | 45 or 60/3 | rV17Gy ≥ 700 mL | Grade 3:9% | Tumor ≥ 35 mm for grade 3 |
| Janoray (13) | Liver mets (35) | +V21Gy < 33% | CP class progression: 4% | |||
| Retrospective | HCC (180) | A, B | 40/5 in CP-A | V20Gy < 20% | Grade 5: 4% | Transaminase ↑, CP score, and platetet count ↓ for grade 5 |
| Sanuki (14) | 35/5 in CP-B | CP score ↑ ≥ 2 points: 11% | ||||
| Current study | HCC (61) | A, B | 54 (36-60)/3 | rV17Gy ≥ 700 mL | ≥ Grade 2: 13% | CP score for ≥ grade 2 |
| Others* (17) | CP class progression: 6% |
Notes
This work was supported by the National Nuclear R&D program of the Ministry of Education, Science and Technology, Republic of Korea (1711022056), and also by the Soonchunhyang University Research Fund (20150001).
AUTHOR CONTRIBUTION Conception and design of the study: Kim MS. Interpretation of data, statistical analysis, and manuscript preparation: Bae SH. Acquisition and interpretation of clinical data: Jang WI. Acquisition and interpretation of dosimetric data: Kim KB, Lee DH. Interpretation of data and revision of the manuscript: Cho CK, Yoo HJ, Han CJ, Park SC. Manuscript approval: all authors.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download